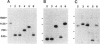Abstract
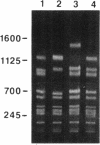
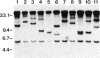
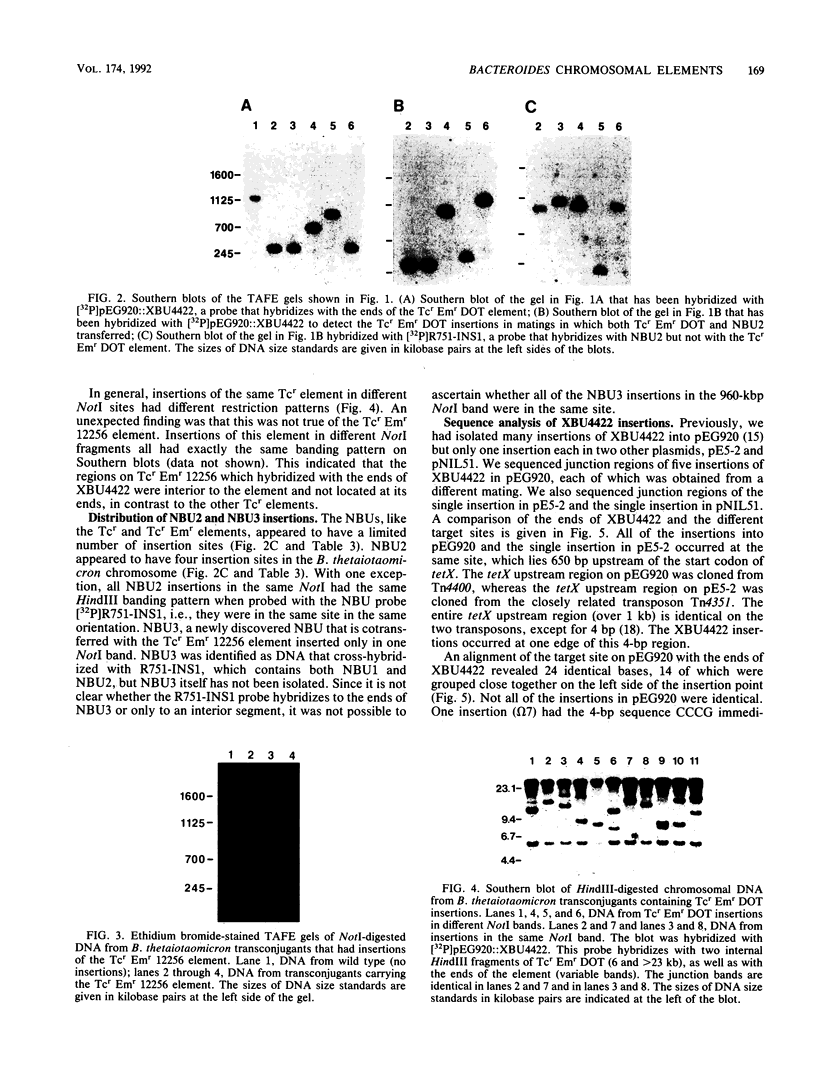
Many strains of Bacteroides harbor large chromosomal elements that can transfer themselves from the chromosome of the donor to the chromosome of the recipient. Most of them carry a tetracycline resistance (Tcr) gene and have thus been designated Tcr elements. In the present study, we have used transverse alternating field electrophoresis to show that all but one of the Tcr elements screened were approximately 70 to 80 kbp in size. The exception (Tcr Emr 12256) was 150 to 200 kbp in size and may be a hybrid element. All of the Tcr elements inserted in more than one site, but insertion was not random. The Tcr elements sometimes cotransfer unlinked chromosomal segments, or nonreplicating Bacteroides units (NBUs). Transverse alternating field electrophoresis analysis showed that insertion of NBUs was not random and that the NBUs did not insert near the Tcr element. Although attempts to clone one or both ends of a Tcr element have not been successful, ends of a cryptic element (XBU4422) were cloned previously and shown to be homologous to the ends of Tcr elements. We have obtained DNA sequences of junction regions between XBU4422 and its target from several different insertions. Comparison of junction sequences with target sequences showed that no target site duplication occurred during insertion and that XBU4422 carried 4 to 5 bp of adjacent chromosomal DNA when it excised from the chromosome and inserted in a plasmid. We identified a short region of sequence similarity between one of the ends of XBU4422 and its target site that may be important for insertion. This sequence contained an 8-bp segment that was identical to the recombinational hot spot sequence on Tn21. XBU4422 could exise itself from plasmids into which it inserted. In most cases, the excision left a single additional A behind in the target site, but precise excision was seen in one case.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Flannagan S. E., Ike Y., Jones J. M., Gawron-Burke C. Sequence analysis of termini of conjugative transposon Tn916. J Bacteriol. 1988 Jul;170(7):3046–3052. doi: 10.1128/jb.170.7.3046-3052.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhstoss S., Richardson M. A., Rao R. N. Site-specific integration in Streptomyces ambofaciens: localization of integration functions in S. ambofaciens plasmid pSAM2. J Bacteriol. 1989 Jan;171(1):16–23. doi: 10.1128/jb.171.1.16-23.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKown R. L., Orle K. A., Chen T., Craig N. L. Sequence requirements of Escherichia coli attTn7, a specific site of transposon Tn7 insertion. J Bacteriol. 1988 Jan;170(1):352–358. doi: 10.1128/jb.170.1.352-358.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier J., Lachapelle J., Couture F., Lafond M., Vézina G., Boissinot M., Levesque R. C. Structural and functional characterization of tnpI, a recombinase locus in Tn21 and related beta-lactamase transposons. J Bacteriol. 1990 Jul;172(7):3745–3757. doi: 10.1128/jb.172.7.3745-3757.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyart-Salmeron C., Trieu-Cuot P., Carlier C., Courvalin P. The integration-excision system of the conjugative transposon Tn 1545 is structurally and functionally related to those of lambdoid phages. Mol Microbiol. 1990 Sep;4(9):1513–1521. doi: 10.1111/j.1365-2958.1990.tb02062.x. [DOI] [PubMed] [Google Scholar]
- Roux K. H., Dhanarajan P. A strategy for single site PCR amplification of dsDNA: priming digested cloned or genomic DNA from an anchor-modified restriction site and a short internal sequence. Biotechniques. 1990 Jan;8(1):48–57. [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- Shoemaker N. B., Anderson K. L., Smithson S. L., Wang G. R., Salyers A. A. Conjugal transfer of a shuttle vector from the human colonic anaerobe Bacteroides uniformis to the ruminal anaerobe Prevotella (Bacteroides) ruminicola B(1)4. Appl Environ Microbiol. 1991 Aug;57(8):2114–2120. doi: 10.1128/aem.57.8.2114-2120.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Barber R. D., Salyers A. A. Cloning and characterization of a Bacteroides conjugal tetracycline-erythromycin resistance element by using a shuttle cosmid vector. J Bacteriol. 1989 Mar;171(3):1294–1302. doi: 10.1128/jb.171.3.1294-1302.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Getty C., Guthrie E. P., Salyers A. A. Regions in Bacteroides plasmids pBFTM10 and pB8-51 that allow Escherichia coli-Bacteroides shuttle vectors to be mobilized by IncP plasmids and by a conjugative Bacteroides tetracycline resistance element. J Bacteriol. 1986 Jun;166(3):959–965. doi: 10.1128/jb.166.3.959-965.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Salyers A. A. A cryptic 65-kilobase-pair transposonlike element isolated from Bacteroides uniformis has homology with Bacteroides conjugal tetracycline resistance elements. J Bacteriol. 1990 Apr;172(4):1694–1702. doi: 10.1128/jb.172.4.1694-1702.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Salyers A. A. Tetracycline-dependent appearance of plasmidlike forms in Bacteroides uniformis 0061 mediated by conjugal Bacteroides tetracycline resistance elements. J Bacteriol. 1988 Apr;170(4):1651–1657. doi: 10.1128/jb.170.4.1651-1657.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer B. S., Bedzyk L., Salyers A. A. Evidence that a novel tetracycline resistance gene found on two Bacteroides transposons encodes an NADP-requiring oxidoreductase. J Bacteriol. 1991 Jan;173(1):176–183. doi: 10.1128/jb.173.1.176-183.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens A. M., Shoemaker N. B., Salyers A. A. The region of a Bacteroides conjugal chromosomal tetracycline resistance element which is responsible for production of plasmidlike forms from unlinked chromosomal DNA might also be involved in transfer of the element. J Bacteriol. 1990 Aug;172(8):4271–4279. doi: 10.1128/jb.172.8.4271-4279.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine P. J., Shoemaker N. B., Salyers A. A. Mobilization of Bacteroides plasmids by Bacteroides conjugal elements. J Bacteriol. 1988 Mar;170(3):1319–1324. doi: 10.1128/jb.170.3.1319-1324.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]