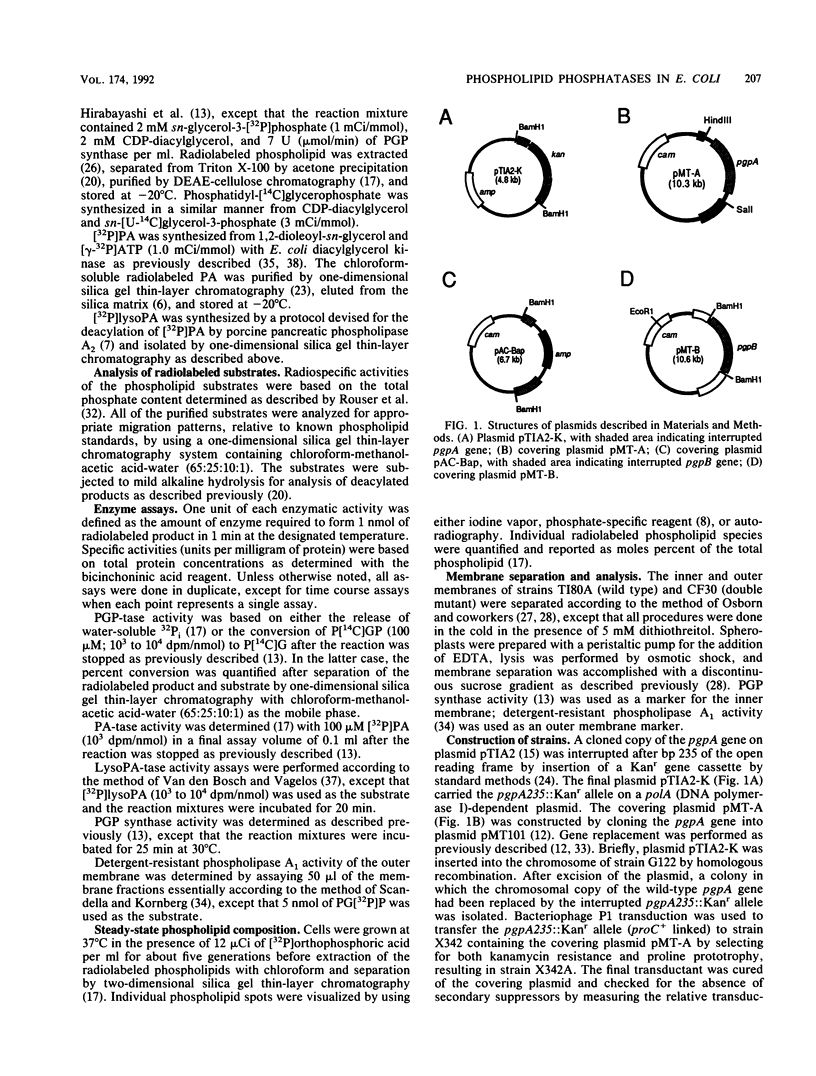
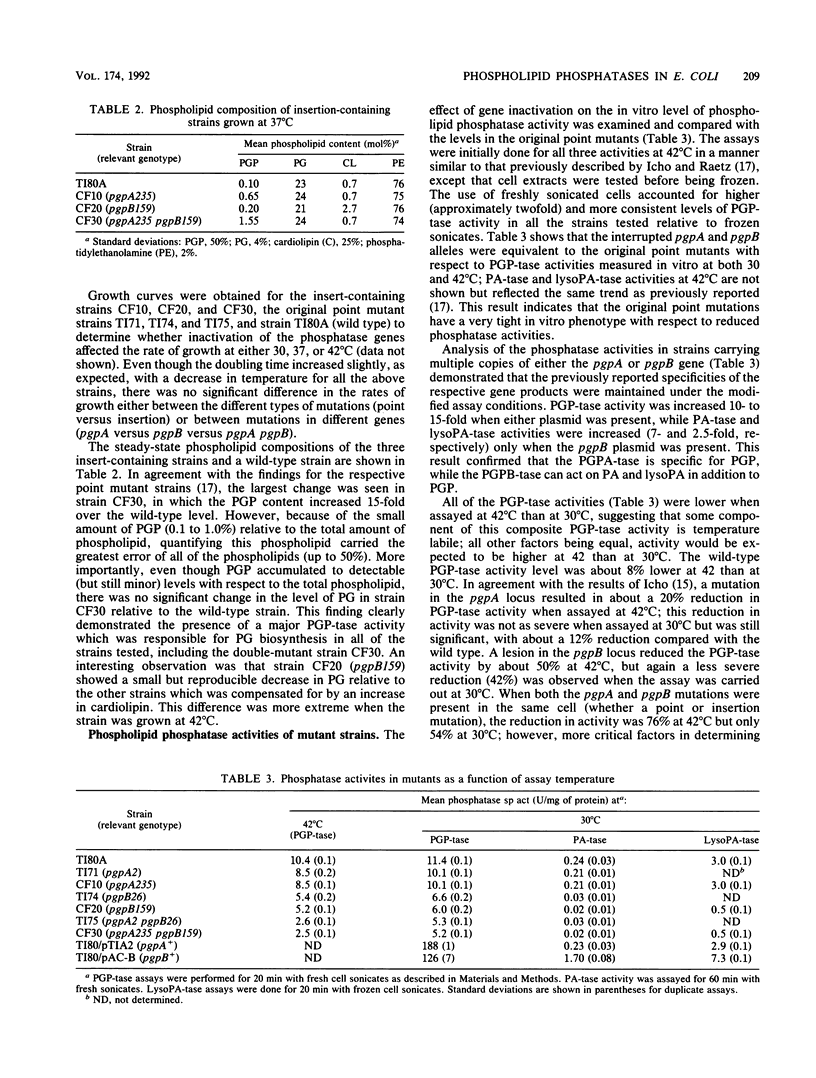
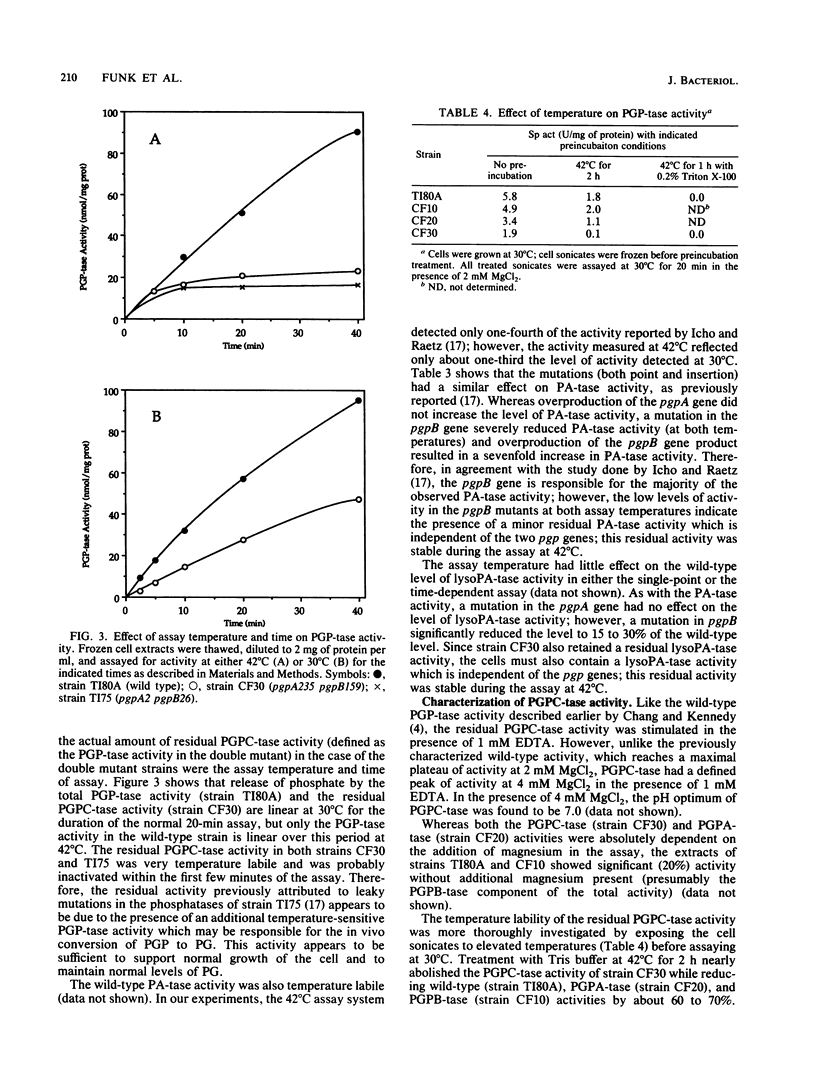
Abstract
To further define the genes and gene products responsible for the in vivo conversion of phosphatidylglycerophosphate to phosphatidylglycerol in Escherichia coli, we disrupted two genes (pgpA and pgpB) which had previously been shown to encode gene products which carried out this reaction in vitro (T. Icho and C. R. H. Raetz, J. Bacteriol. 153:722-730, 1983). Strains with either gene or both genes disrupted had the same properties as the original mutants isolated with mutations in these genes, i.e., reduced in vitro phospholipid phosphatase activities, normal growth properties, and an increase in the level of phosphatidylglycerophosphate (1.6% versus less than 0.1% in wild-type strains). These results demonstrate that these genes are not required for either normal cell growth or the biosynthesis of phosphatidylglycerol in vivo. In addition, the total phosphatidylglycerophosphate phosphatase activity in the doubly disrupted mutant was reduced by only 50%, which indicates that there is at least one other gene that encodes such an activity and thus accounts for the lack of a dramatic effect on the biosynthesis of anionic phospholipids in these mutant strains. The phosphatidic acid and lysophosphatidic acid phosphatase activities of the pgpB gene product were also significantly reduced in gene-interrupted mutants, but the detection of residual phosphatase activities in these mutants indicated that additional genes encoding such phosphatases exist. The lack of a significant phenotype resulting from disruption of the pgpA and pgpB genes indicates that these genes may be required only for nonessential cell function and leaves the biosynthesis of phosphatidylglycerophosphate as the only step in E. coli phospholipid biosynthesis for which a gene locus has not been identified.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. Y., Kennedy E. P. Biosynthesis of phosphatidyl glycerophosphate in Escherichia coli. J Lipid Res. 1967 Sep;8(5):447–455. [PubMed] [Google Scholar]
- Chang Y. Y., Kennedy E. P. Phosphatidyl glycerophosphate phosphatase. J Lipid Res. 1967 Sep;8(5):456–462. [PubMed] [Google Scholar]
- Chattopadhyay P. K., Wu H. C. Biosynthesis of the covalently linked diglyceride in murein lipoprotein of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5318–5322. doi: 10.1073/pnas.74.12.5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- Gopalakrishnan A. S., Chen Y. C., Temkin M., Dowhan W. Structure and expression of the gene locus encoding the phosphatidylglycerophosphate synthase of Escherichia coli. J Biol Chem. 1986 Jan 25;261(3):1329–1338. [PubMed] [Google Scholar]
- Hayashi S., Hara H., Suzuki H., Hirota Y. Lipid modification of Escherichia coli penicillin-binding protein 3. J Bacteriol. 1988 Nov;170(11):5392–5395. doi: 10.1128/jb.170.11.5392-5395.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heacock P. N., Dowhan W. Construction of a lethal mutation in the synthesis of the major acidic phospholipids of Escherichia coli. J Biol Chem. 1987 Sep 25;262(27):13044–13049. [PubMed] [Google Scholar]
- Hirabayashi T., Larson T. J., Dowhan W. Membrane-associated phosphatidylglycerophosphate synthetase from Escherichia coli: purification by substrate affinity chromatography on cytidine 5'-diphospho-1,2-diacyl-sn-glycerol sepharose. Biochemistry. 1976 Nov 30;15(24):5205–5211. doi: 10.1021/bi00669a002. [DOI] [PubMed] [Google Scholar]
- Hirschberg C. B., Kennedy E. P. Mechanism of the enzymatic synthesis of cardiolipin in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Mar;69(3):648–651. doi: 10.1073/pnas.69.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icho T. Membrane-bound phosphatases in Escherichia coli: sequence of the pgpA gene. J Bacteriol. 1988 Nov;170(11):5110–5116. doi: 10.1128/jb.170.11.5110-5116.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icho T. Membrane-bound phosphatases in Escherichia coli: sequence of the pgpB gene and dual subcellular localization of the pgpB product. J Bacteriol. 1988 Nov;170(11):5117–5124. doi: 10.1128/jb.170.11.5117-5124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icho T., Raetz C. R. Multiple genes for membrane-bound phosphatases in Escherichia coli and their action on phospholipid precursors. J Bacteriol. 1983 Feb;153(2):722–730. doi: 10.1128/jb.153.2.722-730.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANFER J., KENNEDY E. P. METABOLISM AND FUNCTION OF BACTERIAL LIPIDS. I. METABOLISM OF PHOSPHOLIPIDS IN ESCHERICHIA COLI B. J Biol Chem. 1963 Sep;238:2919–2922. [PubMed] [Google Scholar]
- KANFER J., KENNEDY E. P. METABOLISM AND FUNCTION OF BACTERIAL LIPIDS. II. BIOSYNTHESIS OF PHOSPHOLIPIDS IN ESCHERICHIA COLI. J Biol Chem. 1964 Jun;239:1720–1726. [PubMed] [Google Scholar]
- Kusters R., Dowhan W., de Kruijff B. Negatively charged phospholipids restore prePhoE translocation across phosphatidylglycerol-depleted Escherichia coli inner membranes. J Biol Chem. 1991 May 15;266(14):8659–8662. [PubMed] [Google Scholar]
- Lill R., Dowhan W., Wickner W. The ATPase activity of SecA is regulated by acidic phospholipids, SecY, and the leader and mature domains of precursor proteins. Cell. 1990 Jan 26;60(2):271–280. doi: 10.1016/0092-8674(90)90742-w. [DOI] [PubMed] [Google Scholar]
- Lin Y. P., Carman G. M. Purification and characterization of phosphatidate phosphatase from Saccharomyces cerevisiae. J Biol Chem. 1989 May 25;264(15):8641–8645. [PubMed] [Google Scholar]
- Nishijima M., Raetz C. R. Membrane lipid biogenesis in Escherichia coli: identification of genetic loci for phosphatidylglycerophosphate synthetase and construction of mutants lacking phosphatidylglycerol. J Biol Chem. 1979 Aug 25;254(16):7837–7844. [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Osborn M. J., Munson R. Separation of the inner (cytoplasmic) and outer membranes of Gram-negative bacteria. Methods Enzymol. 1974;31:642–653. doi: 10.1016/0076-6879(74)31070-1. [DOI] [PubMed] [Google Scholar]
- Pradel E., Boquet P. L. Acid phosphatases of Escherichia coli: molecular cloning and analysis of agp, the structural gene for a periplasmic acid glucose phosphatase. J Bacteriol. 1988 Oct;170(10):4916–4923. doi: 10.1128/jb.170.10.4916-4923.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R., Dowhan W. Biosynthesis and function of phospholipids in Escherichia coli. J Biol Chem. 1990 Jan 25;265(3):1235–1238. [PubMed] [Google Scholar]
- Raetz C. R., Kennedy E. P. Function of cytidine diphosphate-diglyceride and deoxycytidine diphosphate-diglyceride in the biogenesis of membrane lipids in Escherichia coli. J Biol Chem. 1973 Feb 10;248(3):1098–1105. [PubMed] [Google Scholar]
- Rouser G., Fkeischer S., Yamamoto A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970 May;5(5):494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- Russel M., Model P. Replacement of the fip gene of Escherichia coli by an inactive gene cloned on a plasmid. J Bacteriol. 1984 Sep;159(3):1034–1039. doi: 10.1128/jb.159.3.1034-1039.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandella C. J., Kornberg A. A membrane-bound phospholipase A1 purified from Escherichia coli. Biochemistry. 1971 Nov 23;10(24):4447–4456. doi: 10.1021/bi00800a015. [DOI] [PubMed] [Google Scholar]
- Schneider E. G., Kennedy E. P. Phosphorylation of ceramide by diglyceride kinase preparations from Escherichia coli. J Biol Chem. 1973 May 25;248(10):3739–3741. [PubMed] [Google Scholar]
- Schulman H., Kennedy E. P. Relation of turnover of membrane phospholipids to synthesis of membrane-derived oligosaccharides of Escherichia coli. J Biol Chem. 1977 Jun 25;252(12):4250–4255. [PubMed] [Google Scholar]
- Walsh J. P., Bell R. M. sn-1,2-Diacylglycerol kinase of Escherichia coli. Mixed micellar analysis of the phospholipid cofactor requirement and divalent cation dependence. J Biol Chem. 1986 May 15;261(14):6239–6247. [PubMed] [Google Scholar]