Abstract
Glial cells are thought to derive embryologically from either myeloid cells of the hematopoietic system (microglia) or neuroepithelial progenitor cells (astroglia and oligodendrocytes). However, it is unclear whether the glia in adult brains free of disease or injury originate solely from cells present in the brain since the fetal stage of development, or if there is further input into such adult brains from cells originating outside the central nervous system. To test the ability of hematopoietic cells to contribute to the central nervous system, we have transplanted adult female mice with donor bone marrow cells genetically marked either with a retroviral tag or by using male donor cells. Using in situ hybridization histochemistry, a continuing influx of hematopoietic cells into the brain was detected. Marrow-derived cells were already detected in the brains of mice 3 days after transplant, and their numbers increased over the next several weeks, exceeding 14,000 cells per brain in several animals. Marrow-derived cells were widely distributed throughout the brain, including the cortex, hippocampus, thalamus, brain stem, and cerebellum. When in situ hybridization histochemistry was combined with immunohistochemical staining using lineage-specific markers, some bone marrow-derived cells were positive for the microglial antigenic marker F4/80. Other marrow-derived cells surprisingly expressed the astroglial marker glial fibrillary acidic protein. These results indicate that some microglia and astroglia arise from a precursor that is a normal constituent of adult bone marrow.
Keywords: gene transfer, bone marrow transplantation, stem cells, lineage analysis
Besides the cells of the vasculature, the brain comprises two general cell types: neurons and glial cells. Glial cells provide physiological support to neurons and repair neuronal damage due to injury or disease. Macroglia (astroglia and oligodendroglia) are generally considered to be derived from neuroectoderm and are believed to be developmentally distinct from microglia (1). However, the developmental origin of microglia remains debatable (2, 3), the two major views being that they derive either from neuroepithelial cells (4–6) or from hematopoietic cells (i.e. monocytes) (7, 8). The extent to which cells outside the central nervous system (CNS) contribute to the maintenance of microglia in adults remains debatable (compare refs. 9 and 10), and no such contribution to adult neurons or macroglia has been previously described.
To learn if cells of the hematopoietic system are a source of progenitor cells for the CNS, we have used genetically tagged bone marrow cells and monitored their appearance in the brain by in situ hybridization histochemistry (ISHH). We combined ISHH and immunohistochemistry, and performed double-ISHH with digoxigenin and radioactively labeled probes to analyze which cell types might be derived from bone marrow stem cells.
MATERIALS AND METHODS
Gene Transfer and Bone Marrow Transplantation.
Gene transfer into hematopoietic precursors was done as previously described (11, 12), with the addition of stem cell factor to optimize transduction of reconstituting hematopoietic stem cells (13). C57BL/6J mice (The Jackson Laboratory), 6–8 weeks old, were used as donors. Forty-eight hours before marrow harvest, the mice were injected with 5-fluorouracil at a dose of 150 mg/kg to ablate mature blood cells and thereby induce progenitor cells into cycle. Upon harvest, marrow was placed into liquid culture in suspension dishes and grown in DMEM containing 15% fetal bovine serum (BioWhittaker) and supplemented with interleukin 3 (50 ng/ml), interleukin 6 (100 ng/ml), and stem cell factor (100 ng/ml). Growth factors were used to maintain early hematopoietic cells in cycle (13). All were obtained from R & D Systems. After 48 h in culture with growth factors, marrow cells were collected and added to tissue culture dishes containing the F5B producer cell line at subconfluent density. F5B cells shed the N2 retroviral vector, packaged with the ecotropic envelope and carrying the bacterial gene for neomycin resistance (neoR) (14). After 48 h coculture with F5B cells, bone marrow cells were collected by gentle aspiration, suspended to 1 × 107 cells per ml in PBS (in all cases 0.1 M phosphate/140 mM NaCl, pH 7.6) and injected intravenously (2–3 × 106 cells per mouse) via the tail vein into sublethally irradiated (4.5 Gy) female WBB6F1/J-KitW/KitW-v mice. WBB6F1/J-KitW/KitW-v mice are particularly good recipients for bone marrow transplantation because they have genetically defective stem cells (15). This gives normal C57BL/6J donor stem cells a strong repopulating advantage.
In transplants of male donor marrow into female recipients, some marrow was marked with retroviral vector as described. In other cases, marrow was harvested, washed with PBS, and transplanted directly into recipient mice without culturing in growth factor-containing medium or irradiation of recipient animals.
A total of 46 mice were transplanted, 38 with vector-tagged marrow and 8 with male marrow. Five of the transplants with vector-tagged marrow used male donor cells. Mice were sacrificed at various times after transplantation. At least 2 animals were analyzed at each time point, although more were used at the 14-day (n = 10), 35-day (n = 14), and 70-day (n = 6) time points. Tissues were collected and immediately frozen on dry ice for subsequent sectioning. Some animals underwent cardiac perfusion with PBS before tissue harvest. Animals for perfusion were anesthetized with carbon dioxide, then their chests were opened, and PBS was introduced through a cannula placed in the left ventricle. The right atrium was incised to allow release of blood. Animals were perfused with 50 ml of ice-cold PBS over a period of 5 min.
In Situ Hybridization Histochemistry.
Tissues were evaluated with both oligonucleotide and RNA probes. To detect neoR transcripts, two oligonucleotide probes were prepared, complementary to the sequence of the neoR gene either from nucleotides 222–269 or nucleotides 447–494 (numbering with the A of the initiation codon as 1). The oligonucleotides were labeled using terminal transferase (Boehringer Mannheim) and [35S]thio-dATP (New England Nuclear) as described previously (16). An RNA probe, complementary to the entire neoR coding region, was labeled with [35S]thio-UTP using SP6 polymerase (17). Labeling with radioactive probes was detected by dipping hybridized sections in photographic emulsion. Emulsion was exposed for 14 days, then developed and sections were stained, air dried, and coverslipped for microscopic examination. To detect male bone marrow cells transplanted into female recipients, sequences specific to the donor mouse Y chromosome were detected using a complementary RNA probe derived from the plasmid pY353/b (18). Glial fibrillary acidic protein (GFAP) gene expression was detected using an RNA probe complementary to the entire GFAP coding region. The Y chromosome and GFAP probes were labeled using digoxigenin-UTP (19), and digoxigenin labeling was developed for GFAP using alkaline phosphatase as described (19). For detection of the donor Y chromosome, before overnight hybridization with digoxigenin-labeled probes at 55°C, the slides were heated at 90°C for 10 min in hybridization buffer containing the probes to improve access to nuclear DNA. The digoxigenin-labeled Y chromosome was visualized using a modification (20) of an immunostaining amplification method (21), which results in green fluorescein isothiocyanate (FITC) fluorescence.
Twelve-micrometer thick frozen sections were cut in a cryostat, and ISHH was performed as described previously (16, 17). The sections were fixed, dehydrated, and delipidated in ethanol and chloroform, and then hybridization buffer containing the probe(s) was put on the sections. Slides were incubated overnight in a humidified chamber at 37°C (for oligonucleotide probes) or 55°C (for riboprobes).
Nuclear Staining.
To confirm that Y chromosome ISHH coincided with cell nuclei, sections were counterstained with ethidium bromide or 4′,6-diamidino-2-phenylindole (DAPI). Staining was detected by illumination with a mercury lamp using a microscope equipped for fluorescence micrography.
Immunohistochemical Analysis.
For combined ISHH/immunohistochemical analysis, sections were fixed as described previously (22). They then were incubated for 30 min at room temperature in 3% normal goat serum diluted in PBS (containing 0.6% Triton-X 100) to block nonspecific binding. Then, the sections were exposed for 1 h at room temperature to either (i) a polyclonal rabbit antibody that detects the mouse F4/80 monocyte/macrophage marker (23) or (ii) a polyclonal CY-3-labeled rabbit antibody against the astroglial marker GFAP (Sigma) used at a dilution of 1:2,000. Binding of nonlabeled primary antisera was detected with a biotinylated goat anti-rabbit IgG (Jackson ImmunoResearch) diluted 1:500. To detect biotinylated secondary antibody, the sections were incubated for 1 h in an avidin-biotin-peroxidase complex diluted 1:250 in PBS with 0.6% Triton-X 100 (24). The slides then were transferred into 0.1 M Tris·HCl (pH 7.6) and were developed using diaminobenzidine as a substrate. After a thorough wash, the sections were processed for ISHH. Colabeling of cells was determined using a combination of bright-field, polarized, fluorescent, and epi-illumination microscopy. Controls for the immunostaining included leaving out the primary antibodies and using several secondary antibodies (from different species) to confirm that there was no nonspecific binding.
RESULTS
Detection of Donor Cells in the Brain After Bone Marrow Transplantation.
To evaluate the appearance and distribution of donor cells in the brains of recipient mice, animals were sacrificed 3, 5, 7, 14, 28, 35, 42, and 70 days after transplantation with bone marrow cells. At least two animals transplanted with retrovirally tagged marrow were studied at each time point. Mice transplanted with male marrow were analyzed at 35 days (n = 9) and 70 days (n = 4) after transplantation. Using probes specific to the vector neoR transcripts, donor cells were detected beginning with day 3, the earliest time of analysis. Many cells were easily detected throughout the brain by day 7, and cells continued to be detected at all subsequent times. To estimate total number of neoR-positive cells in a brain, every 25th section was collected, and all labeled cells in the sections were counted. The number of labeled cells was multiplied by 25 to arrive at the approximate total number of marked cells in a brain. These calculations showed that the overall number of marrow-derived cells per brain gradually increased with increasing time after transplantation. Three days after transplant, 500 cells were detected per brain. Two to four weeks after transplant the number of cells present had increased to at least 2,000 per brain. In several animals more than 10,000 cells per brain were seen, and in one animal the number of cells was over 30,000.
At 1 week, and occasionally at later times, concentrations of neoR-marked cells were observed in the basal subarachnoid space. Cells marked by the retroviral vector were detected in the hippocampus (Fig. 1A and B), septum (Fig. 1C), and hypothalamus (Fig. 1D). Cells were also detected, among other regions, in the cortex, habenula, pons, and cerebellum (data not shown). Labeled cells were detected after PBS perfusion, indicating that bone marrow-derived cells were an integral part of the brain parenchyma.
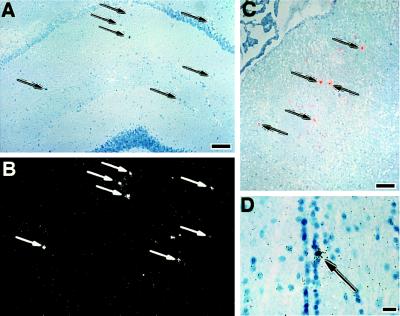
Figure 1.
Detection of donor cells in the brain after bone marrow transplantation with retrovirally tagged bone marrow cells. Arrows indicate representative cells positive by ISHH with 35S-labeled oligonucleotide (A–C) or riboprobe (D). (A and B) Bright (A) and dark (B) field photographs of the same section. ISHH-positive cells (arrows) detected in the hippocampus of an animal 14 days postbone marrow transplantation. (C) Positive cells in the region of the septum of an animal sacrificed 14 days after bone marrow transplantation. The photograph is a double exposure of a bright field image with a dark field image of the same area. The dark field image was photographed using a red filter so that the autoradiographic grains would appear red. (D) A cell (arrow) within the ependyma of the third ventricle. [Bars = 10 μm (A–C) and 40 μm (D).]
Similar regional distribution of donor marrow cells was seen using the Y chromosome probe to detect male donor cells (Fig. 2). Ethidium bromide counter-staining to highlight the nucleus confirmed the nuclear localization of the Y chromosome probe. Many male donor-derived cells were easily detected throughout the brain 35 days after transplantation, and cells continued to be detected at all subsequent times. Cells positive for the Y chromosome marker were detected in the mesencephalon (Fig. 2 A–C), septum (Fig. 2D), striatum (Fig. 2E), and habenula (Fig. 2F). Cells also were detected in the cortex, pons, and cerebellum, among other regions (data not shown). Ex vivo manipulation of the bone marrow cells was not necessary, because male cells were detected in female recipients’ brains even when the transplant was done immediately after marrow harvest.
Figure 2.
Detection of donor cells in several brain regions of a female recipient 6 weeks after transplantation with male bone marrow cells. Arrows indicate representative cells positive for the Y chromosome by ISHH. (A–C) Photomicrographs of a section through the ventral mesencephalon. A is photographed using a rhodamine filter to excite ethidium bromide staining of the nucleus; B is photographed using a FITC filter to excite Y chromosome-specific FITC staining; and C is photographed with a double-pass filter to show overlap of Y chromosome labeling and nucleus-specific ethidium bromide staining. Arrows indicate some of the double-labeled cells. (D–F) Photomicrographs demonstrating Y chromosome positive cells in other brain regions. (D) Septum. (E) Striatum. (F) Habenula. (Bars = 10 μm.)
Several parameters were used to verify that the labeling observed after ISHH was specific. First, no labeling was detected in any tissues of animals transplanted with nonmarked bone marrow cells. That is, without retroviral tagging, probes for the neoR gene exhibited no background labeling, and the Y chromosome probe did not label female tissues. With the Y chromosome riboprobe, we also confirmed that both sense and antisense probes exhibited the same distribution, as expected when hybridizing to chromosomal DNA. The pattern of retrovirally labeled cells was identical in all tissues analyzed, both qualitatively and quantitatively, regardless of which probe was used. Finally, we found donor cells in hematologic organs such as bone marrow and spleen at all time points analyzed (data not shown). The pattern of engraftment was qualitatively similar between retrovirally tagged and male donor cells. However, when female mice were transplanted with retrovirally tagged male marrow, more donor cells were detected with the Y chromosome probe than with the neoR probe. This suggests that not all of the cells migrating from the bone marrow into the brain expressed the retrovirally introduced neoR gene at a level high enough to be detected by ISHH.
Labeling of Brain Sections after ISHH with the Microglial Marker F4/80.
The F4/80 antibody detects the plasma membrane protein F4/80 expressed exclusively on macrophages and microglia (23). Colocalization in brain sections revealed cells labeled by the N2 retroviral vector that also expressed the F4/80 antigen (Fig. 3), confirming that bone marrow-derived cells do contribute to the microglial population in the adult brain. However, only a small percentage of ISHH-positive cells were labeled by immunostaining. Similarly, the minority of antigen-positive cells was doubly labeled by ISHH. The distribution of doubly labeled cells reflected the distribution of cells labeled only by ISHH or by immunoshistochemistry, i.e., they were widely distributed throughout the brain.
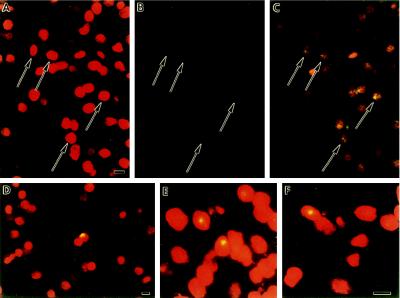
Figure 3.
Double-labeling of brain sections detects cells coexpressing the microglial marker F4/80 and the neoR retroviral tag. The F4/80 monocyte/macrophage antigen was detected by indirect immunofluorescent antibody labeling; 35S-radiolabeled probes were used to hybridize to neoR mRNA. The photomicrograph is of a representative field from an animal sacrificed 35 days after bone marrow transplantation. A cell in the center stains positive for the F4/80 antigen (red) and exhibits labeling with radioactive probe to neoR transcripts. The dark field image was photographed using a green filter so that autoradiographic grains would appear green (yellow where they overlap red immunostaining). (Bar = 10 μm.)
Labeling of Brain Sections for Both the Astroglial Marker GFAP and the neoR Retroviral or Y Chromosome Donor Cell Tag.
The ISHH-positive, F4/80 negative cells could be cells of the myeloid lineage that had not differentiated to express the F4/80 antigen. Or, they could represent a contribution of bone marrow-derived cells to other than myeloid cell lineages. To distinguish between these alternative possibilities, ISHH-positive cells were examined for the expression of another lineage marker, GFAP, specific for astroglia. Surprisingly, we found occasional cells (Fig. 4A) which were labeled both by ISHH (for the donor marrow neoR marker) and by indirect immunohistochemistry (for GFAP). Counting all of the donor cells present in every 25th section obtained from recipient mice 4 weeks after transplantation (n = 3), we calculated that as many as 3 × 104 neoR-marked donor cells were present per brain. Of that total donor cell number, we estimated between 0.5% and 2% exhibited GFAP expression.
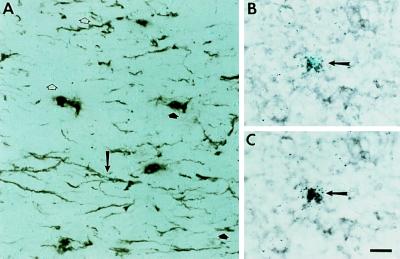
Figure 4.
Double-labeling of brain sections detects cells coexpressing the astroglial marker GFAP and the neoR retroviral tag. (A) Detection of cells within the optic tract expressing GFAP protein using peroxidase-based immunohistochemical staining combined with ISHH to detect expression of neoR transcripts. The arrow indicates a double-labeled cell. Open arrowheads indicate clusters of grains indicative of a neoR-marked cell that is not expressing GFAP. Filled arrowheads indicate GFAP-positive positive cells that are not marked with the retroviral tag. (B and C) Detection of GFAP transcripts by ISHH using digoxigenin-labeled probes combined with detection of neoR transcripts by ISHH using 35S-labeled probes. The photograph is of a section through the cerebral cortex. (B) Polarized epifluorescent illumination to emphasize grains indicative of hybridization with 35S-labeled probe for neoR. (C) Bright field illumination emphasizing digoxigenin staining of GFAP transcripts. The cell indicated by the arrow is double-labeled. (All photomicrographs are at the same magnification. Bar = 20 μm.)
To confirm that GFAP mRNA was present in some neoR-positive cells, we also did double-ISHH analysis. Cells coexpressing GFAP and neoR mRNAs were identified using a digoxigenin-labeled riboprobe against GFAP mRNA together with a 35S-labeled probe for the neoR gene marking the donor marrow. As illustrated in Fig. 4 B and C, we found cells labeled with both probes. Their frequency was approximately equal to the frequency of the ISHH/GFAP immunostained double cells.
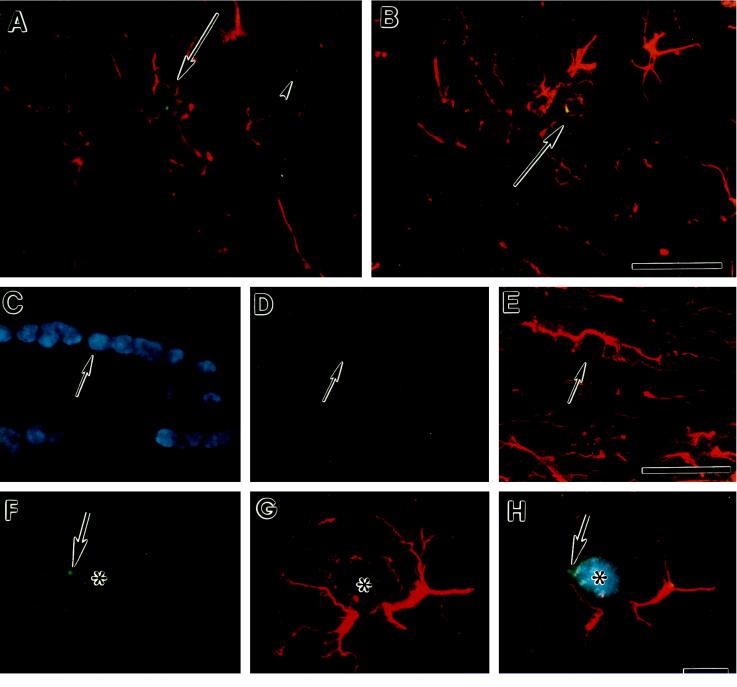
We also found doubly labeled cells in multiple animals when ISHH to detect male cells with the Y chromosome marker was combined with immunohistochemistry to detect GFAP protein (Fig. 5). Using DAPI staining to highlight the nucleus and three-channel photomicrography, we confirmed that the Y-chromosome ISHH was associated with the nuclei of GFAP-positive cells (Fig. 5 C–H).
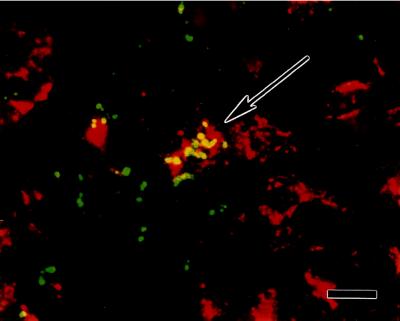
Figure 5.
Male bone marrow derivative cells express GFAP. Photomicrographs are of double-labeled cells found in the brains of two different female recipient mice 10 weeks after bone marrow transplantation. Male donor cells were detected with a Y chromosome-specific riboprobe as described in Fig. 1. Astroglia were identified using a CY3-labeled polyclonal antibody against the astroglial marker GFAP. (A and B) Double-labeled cells are indicated by arrows. The arrowhead in A points to a Y chromosome-positive cell derived from the male donor marrow that is not GFAP immunopositive. A is a section through the cortex. B is a section through the septum. (Bar in B applies to A and B and equals 20 μm.) (C, D, and E) Photomicrographs of a section through the corpus callosum. In C, the section is illuminated with ultraviolet light to excite DAPI fluorescent staining of the nucleus. Nuclei from all cells are stained. D is illuminated to excite FITC staining of the Y chromosome. E is illuminated to excite CY3-immunostaining of GFAP. A Y chromosome/GFAP double labeled cell is indicated by the arrow. (Bar in E applies to C–E and equals 20 μm.) (F, G, and H) Photomicrographs of a single field from a section through the amygdala. F illustrates the green FITC staining associated with the Y chromosome. G shows the red GFAP immunostaining, and H is a double-exposure of the same field, first with a double band pass filter to excite FITC and CY3 fluorescence, then with ultraviolet illumination to excite the blue DAPI fluorescent staining of the nucleus (*). The green Y chromosome fluorescence is indicated by the arrow (F and H). (Bar in H applies to F–H and equals 4 μm.) Sections in A and C–E were obtained from one mouse, while those in B and F–H were obtained from another.
DISCUSSION
The results reported here confirm that cells derived from the bone marrow can migrate into the brains of adult mice. Furthermore, we have found that this migration is rapid, with numerous cells present by the third day after transplant. These new cells are distributed throughout the brain, and appear to reside within the parenchyma, because perfusion with PBS does not remove them. Occasional donor marrow-derived cells were found in association with vascular structures. Moreover, densities of donor cells in the parenchyma paralleled the capillary density of a given region. For instance, cortex, with fewer capillaries, had a lower cell density than the more vascularized choroid plexus. Regions with a higher capillary density, such as the area postrema, also had the highest density of marrow-derived cells within the parenchyma.
Double-labeling analyses show that at least some bone marrow-derived cells acquire microglial antigenic markers. However, we also observed many cells positively labeled by ISHH that did not express the F4/80 antigen. This may be due simply to a level of antigen below the limits of detection in our assay. Alternatively, it is possible that the F4/80 marker is expressed on marrow-derived cells only after they fully differentiate into microglia, while less mature microglial precursors are not recognized by the antibody to F4/80. Nonetheless, our results strongly support the view that hematopoietic cells outside the CNS contribute to the maintenance of microglia in healthy adults. While a partial CNS origin of adult microglia cannot be excluded, our data is inconsistent with an exclusively CNS origin. Moreover, although our experiments did not examine fetal origins of microglia, the finding of hematopoietically derived microglia in healthy adults is also consistent with a hematopoietic origin of microglia in development.
Surprisingly, we found that some hematopoietic cells (tagged either with a retroviral vector or by transplant of male cells into a female recipient) give rise to cells other than microglia, specifically to cells that exhibit astroglial markers. Although this observation is unexpected, it is based on identical results in multiple animals using two independent means of cell tagging with both cytoplasmic and nuclear markers.
The appearance of marrow-derived astroglia seems a normal process in these animals. Because marrow-derived cell numbers detected in the brain increased over time, their appearance does not appear to be a consequence of the transplantation procedure itself. If appearance in the brain was a byproduct of transplantation, one might expect tagged cell numbers in the brain to peak and then decline, which was not observed. Rather, the data is consistent with existence of cells, among the populations of marrow-engrafting cells, capable of continuous generation of progenitors that migrated to the brain. Interestingly, cells with marrow markers were seen in the ventricular ependyma (Fig. 1D). In fact, in many animals, marrow-derived cells could be found concentrated subependymally (unpublished data). The subependymal zone is an important source of neuronal and glial progenitors during development (25–27) and in adults (28, 29). Finding bone-marrow derived cells in this location opens the possibility that such cells receive cues guiding their differentiation once they enter the brain. Studies evaluating this possibility are ongoing.
No obvious pathology such as gliosis was detected in the brain of any transplant recipient (n = 46). Some recipient animals were irradiated before receiving bone marrow transplants to see if marrow purging enhanced engraftment and seeding of implanted cells. However, radiation dosages were at least one order of magnitude below those known to induce pathological changes in the CNS (30). Indeed, we found preconditioning of recipients was not necessary. Male donor cells engrafted and persisted for at least 10 weeks even without irradiation (Fig. 5 C–H). Furthermore, as many Y chromosome/GFAP double-positive cells were seen with as without irradiation. The wide distribution of GFAP-positive cells in both gray and white matter suggests that bone marrow-derived progenitors are not restricted to differentiate into a particular subclass of astroglia. That is, marrow-marked cells contributed to both fibrous astrocytes in the white matter (Figs. 4A and 5 C–E) and protoplasmic astrocytes in the gray matter (Figs. 4 B and C and 5A).
One alternative explanation for our observing GFAP staining of cells bearing marrow markers is that processes from endogenous astroglia surround the in-migrating cells from the donor marrow. However, some of our data argue against this possibility. First, cytoplasmic neoR ISHH labeling coincided with cytoplasmic GFAP immunostaining (Fig. 4A). Furthermore, upon evaluation of 50 to 100 male nuclei associated with GFAP staining, no nuclei were seen that could be considered part of an engulfing astroglial cell. If endogenous astroglia were the source of the GFAP staining associated with donor male nuclei, one might expect the geometry in 12 μm sections to reveal the cell body and nucleus corresponding to the putative engulfing processes in at least a few cases. After analyzing dozens of sections, no such cases were observed. Culturing astroglia obtained from brains of transplant recipients may help to resolve this issue in the future.
Because only about 10% of marrow-derived cells in the brain exhibit expression of either the microglial F4/80 antigen or the astroglial marker GFAP, the identity of the majority of bone marrow-derived cells remains an open question. Nonetheless, there is clearly a measurable contribution by cells of hematopoietic origin to the glial cell population of the brain in adult mice, which suggests that some glial progenitors reside outside the CNS. The observation of marrow-derived astroglia in the optic tract (Fig. 4A) suggests that some of these marrow-derived progenitors may be similar to the previously recognized astroglial precursor (31). Alternatively, two classes of astroglia may exist, one arising from the previously recognized neuroepithelial astroglial precursor present in the brain from birth, and another from a different precursor that is a normal constituent of adult bone marrow. It would be interesting to determine if astroglia arising from the different sources of progenitors also exhibit different functional characteristics.
Microglia and astroglia respond differently to brain injury. In fact, astrogliosis often appears to be a response to primary microgliosis (32, 33). There is also evidence that different brain lesions elicit different microglial and astroglial responses (34). Our results suggest that gene transfer into hematopoietic progenitors can be used to introduce genes into microglia and astroglia that then might participate in the gliosis associated with a CNS pathology. The detection of marrow-derived cells in brains within days of transplantation implies that genetically altered hematopoietic cells could be used to treat acute diseases of the brain.
Although many neurotrophic factors show promise in the treatment of CNS disorders, their use has been hindered by their inability to cross the blood-brain barrier and by their limited diffusion into CNS tissues (35). In addition, adverse effects have been reported after systemic administration of some neurotrophins (36). Using marrow-derived cells to deliver therapeutic proteins directly to the site of CNS pathology may be more benign than systemic administration of toxic molecules. In addition, using vectors with cell type-specific promoters could restrict gene expression specifically to reactive astroglia or microglia, thereby providing greater therapeutic precision for gene therapy of CNS disease.
Acknowledgments
We are grateful to Gyöngyi Harta for her skillful technical assistance, and to Ricardo Dreyfuss for his expert help with the photomicrography. We also thank Drs. Miklos Palkovits, Henry D. F. Webster and Beth J. Hoffman for their comments on the manuscript. We also appreciate the generosity of Dr. Siamon Gordon in providing the rabbit polyclonal antiserum against F4/80.
ABBREVIATIONS
- ISHH
in situ hybridization histochemistry
- neoR
neomycin resistance
- GFAP
glial fibrillary acidic protein
- CNS
central nervous system
- FITC
fluorescein isothiocyanate
- DAPI
4′,6-diamidino-2-phenylindole
References
- 1.Skoff R P, Knapp P E. In: Neuroglia. Kettenmann H, Ransom B R, editors. New York: Oxford Univ. Press; 1995. pp. 135–148. [Google Scholar]
- 2.Theele D P, Streit W J. Glia. 1993;7:5–8. doi: 10.1002/glia.440070104. [DOI] [PubMed] [Google Scholar]
- 3.Altman J. Trends Neurosci. 1994;17:47–49. doi: 10.1016/0166-2236(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 4.Lewis P D. Brain. 1968;91:721–738. doi: 10.1093/brain/91.4.721. [DOI] [PubMed] [Google Scholar]
- 5.Kitamura T, Miyake T, Fujita S. J Comp Neurol. 1984;226:421–433. doi: 10.1002/cne.902260310. [DOI] [PubMed] [Google Scholar]
- 6.Neuhaus J, Fedoroff S. Glia. 1994;11:11–17. doi: 10.1002/glia.440110104. [DOI] [PubMed] [Google Scholar]
- 7.Perry V H, Gordon S. Trends Neurosci. 1988;11:273–278. doi: 10.1016/0166-2236(88)90110-5. [DOI] [PubMed] [Google Scholar]
- 8.Ling E-A, Wong W-C. Glia. 1993;7:9–18. doi: 10.1002/glia.440070105. [DOI] [PubMed] [Google Scholar]
- 9.Perry V H. Macrophages and the Nervous System. Austin, TX: Landes; 1994. [Google Scholar]
- 10.Fedoroff S. In: Neuroglia. Kettenmann H, Ransom B R, editors. New York: Oxford Univ. Press; 1995. pp. 162–181. [Google Scholar]
- 11.Eglitis M A, Kantoff P, Gilboa E, Anderson W F. Science. 1985;230:1395–1398. doi: 10.1126/science.2999985. [DOI] [PubMed] [Google Scholar]
- 12.Bodine D M, Seidel N, Karlsson S, Nienhuis A W. Prog Clin Biol Res. 1990;352:287–299. [PubMed] [Google Scholar]
- 13.Luskey B D, Rosenblatt M, Zsebo K, Williams D A. Blood. 1992;80:396–402. [PubMed] [Google Scholar]
- 14.Armentano D, Yu S-F, Kantoff P W, von Ruden T, Anderson W F, Gilboa E. J Virol. 1987;61:1647–1650. doi: 10.1128/jvi.61.5.1647-1650.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russell E S. Adv Genet. 1979;20:357–459. [PubMed] [Google Scholar]
- 16.Young W S, Mezey E, Siegel R E. Brain Res. 1986;387:231–241. doi: 10.1016/0169-328x(86)90029-x. [DOI] [PubMed] [Google Scholar]
- 17.Bradley D J, Towle H C, Young W S. J Neurosci. 1992;12:2288–2302. doi: 10.1523/JNEUROSCI.12-06-02288.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bishop C E, Boursot P, Baron B, Bonhomme F, Hatat D. Nature (London) 1985;315:70–72. doi: 10.1038/315070a0. [DOI] [PubMed] [Google Scholar]
- 19.LeMoine C, Young W S. Proc Natl Acad Sci USA. 1992;89:3285–3289. doi: 10.1073/pnas.89.8.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunyady B, Krempels K, Harta G, Mezey E. J Histochem Cytochem. 1996;44:1353–1362. doi: 10.1177/44.12.8985127. [DOI] [PubMed] [Google Scholar]
- 21.Berghorn K A, Bonnett J H, Hoffman G E. J Histochem Cytochem. 1994;42:1635–1642. doi: 10.1177/42.12.7983364. [DOI] [PubMed] [Google Scholar]
- 22.Lawson L J, Perry V H, Dri P, Gordon S. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- 23.Austyn J M, Gordon S. Eur J Immunol. 1981;11:805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- 24.Hsu S M, Raine L, Fanger H. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- 25.Smart I. J Comp Neurol. 1961;116:325–347. [Google Scholar]
- 26.Altman J. J Comp Neurol. 1969;137:433–458. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- 27.Sturrock R R, Smart I H M. J Anat. 1980;130:391–415. [PMC free article] [PubMed] [Google Scholar]
- 28.Alvarez-Buylla A, Lois C. Stem Cells (Dayton) 1995;13:263–272. doi: 10.1002/stem.5530130307. [DOI] [PubMed] [Google Scholar]
- 29.Weiss S, Reynolds B A, Vescovi A L, Morshead C, Craig C G, van der Kooy D. Trends Neurosci. 1996;19:387–393. doi: 10.1016/s0166-2236(96)10035-7. [DOI] [PubMed] [Google Scholar]
- 30.Chiang C S, McBride W H, Withers H R. Radiother Oncol. 1993;29:60–68. doi: 10.1016/0167-8140(93)90174-7. [DOI] [PubMed] [Google Scholar]
- 31.Lillien L E, Raff M C. Neuron. 1990;5:111–119. doi: 10.1016/0896-6273(90)90301-u. [DOI] [PubMed] [Google Scholar]
- 32.Giulian D. In: The Biochemical Pathology of Astrocytes. Norenberg M D, Hertz L, Schoustoe A, editors. New York: Liss; 1988. pp. 91–105. [Google Scholar]
- 33.Giulian D, Chen J, Ingeman J E, George J K, Noponen M. J Neurosci. 1989;9:4416–4429. doi: 10.1523/JNEUROSCI.09-12-04416.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson M A, Molliver M E. Glia. 1994;11:18–34. doi: 10.1002/glia.440110105. [DOI] [PubMed] [Google Scholar]
- 35.Lindsay R M, Wiegand S J, Altar C A, DiStefano P S. Trends Neurosci. 1994;17:182–190. doi: 10.1016/0166-2236(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 36.Verrall M. Nature (London) 1994;370:6. doi: 10.1038/370006a0. [DOI] [PubMed] [Google Scholar]