Abstract
Studies of the location of somatosensory and auditory cortical responses have shown anomalous hemispheric asymmetries in a variety of neurodevelopmental disorders. Although to date, abnormal asymmetries in the somatosensory region have shown greater specificity being reported only in psychotic adults. This study examines functional organization of somatosensory cortices using magnetoencephalography in adolescents with childhood-onset psychotic disorders. Eighteen young outpatients with history of psychotic illness and 15 healthy adolescents participated. Both groups underwent stimulation of the index finger as magnetoencephalography was acquired from the contralateral hemisphere. Neural generators of the M50 somatosensory response were modeled using an equivalent current dipole for each hemisphere, and later investigated for systematic variation with diagnosis. Consistent with adult psychosis data, adolescent patients showed hemispheric symmetry in the anterior-posterior dimension. In controls, a reversed pattern of hemispheric asymmetry was observed relative to previous findings in normal adults (Reite et al., 2003), but trend-level correlations suggested source location became more adult-like during transition from adolescence to adulthood. Source parameters also exhibited robust inter-hemispheric correlations only in adolescent controls. In sum, source locations, patterns of cerebral lateralization, and inter-hemispheric correlations all distinguish patients from their normally developing cohort. These findings suggest aberrant maturation underlies the reduction in cerebral laterality associated with psychosis.
Keywords: schizophrenia, somatosensory, bipolar, MEG, asymmetry, development
1. Introduction
Magnetoencephalography (MEG) studies have repeatedly shown the location of auditory cortical responses to be abnormal in both children and adults diagnosed with distinct neurodevelopmental disorders (Reite et al., 1989, 1997; Rojas et al., 1997, 2001; Teale et al., 2000; Rockstroh et al., 2001; Heim et al., 2003a, 2003b, 2004; Paul et al., 2006). In normal participants, auditory responses most often exhibit inter-hemispheric asymmetry, being further anterior in the right relative to left hemisphere. However, in adults with fragile X syndrome (Rojas et al., 2001), schizophreniform disorders (Reite et al., 1989, 1997; Hajek et al., 1997a; Rojas et al., 1997; Tiihonen et al., 1998; Teale et al., 2000; Rockstroh et al., 2001), or developmental dyslexia (Heim et al., 2003a, 2004) such activated cortical areas fail to exhibit this asymmetry, being either asymmetrically reversed (left anterior to right) or statistically symmetrical. Beyond the auditory cortex, several studies have reported similar findings for the somatosensory region in patients with schizophreniform or mood-related psychosis. The somatosensory strip also follows a right anterior to left functional asymmetry in normal adults (Wikstrom et al., 1997), yet MEG studies have shown adults with psychosis exhibit reversed asymmetry reminiscent of the disorganization observed in auditory cortices of comparable patients (Reite et al., 1999a, 1999b, 2003).
Interestingly, previous investigations have shown aberrant somatosensory organization in adults with schizoaffective disorder (Reite et al., 1999b), schizophrenia (Reite et al., 2003), and psychotic but not non-psychotic mood disorders (Reite et al., 1999a). In fact, to date, positive findings in other psychiatric conditions not accompanied by psychosis have not been reported, which could indicate a greater degree of diagnostic specificity than that observed in the auditory system or merely a paucity of applicable studies. In addition, there are currently no published reports investigating such variables in healthy adolescents, nor those with earlier-onset psychoses (i.e., < 13 years of age at onset). Thus, whether normal patterns of asymmetry (right anterior to left) will be observed in younger participants remains to be demonstrated, as does the potential connection between psychoses with early-onset and anomalous functional asymmetry in somatosensory cortices.
A variety of neuronal metrics have indicated normal patterns of adult asymmetry may not be fully developed until late adolescence or even early adulthood. For example, it is well appreciated that the latency of both auditory and visual cortical responses change as a function of development (Auditory: Tonnquist-Uhlen et al., 1995; Rojas et al., 1998; Visual: Crognale et al., 2001), and recently hemispheric asymmetries have been discerned with this index. Gage and colleagues (2003) reported the maturation–mediated decrease in M100 peak latency, for auditory responses, decreases on a sharper slope in the dominant hemisphere relative to the non-dominant hemisphere from 8- to 16-years of age. Additionally, there is an EEG study using the indexes of intra-hemispheric phase and coherence in a large sample containing infants through young adults, which clearly demonstrated the two cerebral hemispheres normally develop along similar overall trajectories, but with different postnatal onset times and at distinct maturational rates (Thatcher et al., 1987). Recent investigations of structural maturation have been largely consistent with these physiological observations. Several studies have indicated most brain regions exhibit a progressive age-related reduction in gray matter density or thickness throughout adolescence (Sowell et al., 2003, 2004; Giedd et al., 2004; Gogtay et al., 2004), which apparently follows an asymmetrical course with neural areas of the dominant hemisphere developing before their non-dominant homologues (Gogtay et al., 2004). In light of these findings, an examination of functional asymmetries in the healthy and diseased adolescent brain may enhance the field’s understanding of anomalous asymmetry patterns observed in psychotic adults.
In the current study, an innocuous tactile stimulus was used to elicit M50 neuromagnetic responses from the somatosensory cortices in a group of normal adolescents and a group with early-onset psychoses, comprised of both schizophreniform and psychotic mood disorders. The tactile response evoked through this procedure appears to be generated in the hand region of the primary-somatosensory cortex (i.e., area 3b of the postcentral gyrus; Kakigi et al., 2000; Hoechstetter et al., 2001), and prior MEG studies utilizing the single equivalent-current-dipole (ECD) model have uniformly estimated the M50 source as somatotopically-organized S1 cortices (Yang et al., 1993; Iguchi et al., 2001). Primary comparisons were between the larger psychotic group, including both schizophreniform and mood related psychoses, and a matched-sample of never mentally ill adolescents. Comparing across specific diagnoses is justified in the sense that there are likely biological markers shared in common among specific manifestations of psychosis which relate more directly to psychosis (e.g., positive symptoms) than to other phenotypes (e.g., negative symptoms, mood instability), and prior studies in adults have suggested somatosensory cortical asymmetries may more closely approximate this type of marker. Indeed, as noted above, these studies have shown normal patterns of somatosensory asymmetry in non-psychotic mood disorder patients, along with similarly anomalous patterns in those with schizophreniform or mood-related psychosis (Reite et al., 1999a, 1999b, 2003). Other evidence has indicated particular delays in motor, linguistic, and social development may hold some predictive capacity for the incidence of (non-specific) psychoses later in life (Sigurdsson et al., 1999; Isohanni et al., 2001). Nevertheless, in addition to main comparisons, preliminary contrasts were performed between mood related and schizophreniform psychoses.
In healthy adolescents we hypothesized MEG source parameters of peak latency and strength to correlate strongly between the two hemispheres, and for parameter means to more closely approximate adult patterns in the left hemisphere. On the contrary, the coordination between right- and left-hemispheric developmental processes may be reduced or even disengaged in early-onset psychosis, as such an anomalous pattern of maturation could underlie the association between delayed cerebral dominance and later incidence of psychosis (Crow et al., 1996). Accordingly, in psychotic adolescents these source parameters were expected to be less correlated between the hemispheres, thereby suggesting a lack of coordinated developmental processes. In regard to hemispheric asymmetries in source location, M50 generators were hypothesized to be more symmetrical in adolescents with psychosis relative to matched controls (i.e., asymmetry patterns reminiscent of those observed in adult studies).
2. Methods and materials
2.1. Subjects
Eighteen adolescents with psychosis (10 male) and 15 age- and education-matched comparison subjects (10 male) were studied. All subjects were right-handed as determined by the Annett Handedness Scale (Annett, 1985). Additional demographic information is provided in Table 1. Of the 18 patients, eight were diagnosed with a psychotic mood disorder (Bipolar I Disorder with psychotic features (n = 7); Major Depressive Disorder with psychotic features (n = 1)), and the remaining 10 had a schizophreniform disorder (Schizophrenia (n = 7); Schizophreniform (n = 1); Schizoaffective (n = 1); Psychosis NOS (n = 1)). All patients were in outpatient treatment, medicated, and referred by their doctor. Seventeen of the 18 patients were taking medications for which chlorpromazine equivalency has been reported (Centorrino et al., 2002a; Woods, 2003), and all were taking multiple medications at the time of study (see Supplementary Materials). Upon entrance into this study, all participants in the psychotic group were re-diagnosed using the Schedule for Affective Disorders and Schizophrenia for School-Age Children – Present and Lifetime Version (K-SADS-PL; Kaufman et al., 1997), and a review of medical records. Exclusionary criteria included any medical illness affecting CNS function, confounding/dual diagnoses, neurological disorder, history of head trauma, and current substance abuse. Comparison subjects met the same exclusionary criteria, but had no personal or first-degree family history of an Axis I diagnosis. All control participants were recruited from the community and evaluated upon enrollment using the K-SADS-PL. Informed consent was obtained in accord with the guidelines of the Colorado Multiple Institutional Review Board.
Table 1.
Demographics and MEG Source Parameters
| Variable | NC* | PMD* | SD* | t-Statistic (df) | P-value |
|---|---|---|---|---|---|
| Age | 13.26 (2.0) | 13.21 (2.6) | 15.03 (2.8) | 1.30 (31) | P=0.21 |
| Handedness | 0.79 (0.17) | 0.89 (0.19) | 0.78 (0.21) | 0.52 (31) | P=0.61 |
| Education (years) | 7.33 (1.45) | 7.30 (1.27) | 8.10 (1.56) | 0.91 (31) | P=0.37 |
| Age at onset✞ | NA | 11.00 (2.52) | 9.64 (4.28) | 0.85 (16) | P=0.41 |
| L fit-interval (ms) | 17.37 (6.6) | 19.83 (5.3) | 22.50 (8.8) | 1.60 (31) | P=0.12 |
| R fit-interval (ms) | 19.81 (6.0) | 20.07 (7.5) | 21.63 (9.7) | 0.43 (31) | P=0.67 |
| L latency (ms) | 47.56 (8.6) | 50.84 (5.6) | 50.29 (6.1) | 1.80 (31) | P=0.25 |
| R latency (ms) | 46.80 (7.0) | 47.84 (6.3) | 57.02 (12.5) | 1.86 (31) | P=0.07 |
| L amplitude (nAm) | 4.39 (1.5) | 4.64 (1.5) | 5.96 (6.1) | 0.79 (31) | P=0.44 |
| R amplitude (nAm) | 4.79 (1.3) | 5.53 (2.6) | 4.87 (2.7) | 0.51 (31) | P=0.62 |
| L GOF | 97.70 (2.0) | 98.85 (0.4) | 97.59 (1.8) | 0.74 (31) | P=0.47 |
| R GOF | 98.26 (1.3) | 97.71 (1.4) | 96.85 (2.1) | 1.84 (31) | P=0.08 |
| X-asym index | -7.50 (6.5) | -2.51 (4.5) | -2.07 (10.0) | 2.06 (31) | P=0.05 |
| Y-asym index | -3.39 (8.6) | -0.05 (5.1) | -2.24 (14.2) | 0.62 (31) | P=0.54 |
| Z-asym index | -2.65 (5.4) | -0.38 (4.1) | -0.66 (11.7) | 0.80 (31) | P=0.43 |
|
| |||||
NA: Not applicable
NC: Normal controls
PMD: Psychotic mood disorder
SD: Schizophreniform disorder
t and P values reflect controls vs. patients (PMD+SD) unless otherwise specified
Values are mean (SD)
t and P values reflect PMD vs. SD groups
2.2. Stimuli
A single pneumatic pressure pulse was presented to the volar distal phalanx of the first digit by means of a 1-cm-diameter rubber bladder encased in plastic housing lightly taped to the finger (4-D Neuroimaging, San Diego, CA, USA). The duration of a single stimulation was 200 ms with a constant inter-stimulus-interval of 1 s. For each hemisphere, at least 1000 trials were collected. The time of bladder inflation onset was adjusted to correspond to time zero in the recording window, and all recordings were made from the hemisphere contralateral to the hand stimulated.
2.3. MEG recordings
Magnetic field data were obtained with a 37-channel Magnes I biomagnetometer (4-D Neuroimaging), which was equipped with concentric rings of first-order axial gradiometers (coil diameter = 2 cm, baseline = 5 cm). Contralateral recordings were made with subjects lying on a nonmagnetic bed within the magnetically-shielded room. To maintain alertness during recordings, all subjects watched a self-selected movie on a monitor about 4 m distant. Data were collected over a 250 ms window, including a 50 ms pre-stimulus period, using a 16-bit analog-to-digital converter with a sampling rate of 1041.7 Hz. Analog filters were set at 200 Hz low-pass and 1 Hz high-pass during all data acquisitions. The Magnes SCP software (Version 1.6) was used for all recordings, and the three fiducial points were determined using a Polhemus 3SPACE digitizer (Colchester, VT, USA).
Coil locations and orientations were then expressed in the coordinate system defined as follows: Y-axis along the line between the preauricular points with positive to the left, X-axis perpendicular to the Y-axis at the midpoint with positive values toward the nasion, and Z-axis perpendicular to the same plane with positive values starting at the midpoint and proceeding in the upward direction. To precisely position the instrument for recording, the field topography was inspected using the building average and the 50 ms post-stimulus component was required to have a balanced distribution with equal numbers of ingoing and outgoing field measurements across the array. If this condition was not initially met, the magnetometer was repositioned and the recording repeated.
2.4. MEG analysis
Following MEG acquisition, all data files were visually edited trial-by-trial for eye-blink, head movement, and other artifacts before averaging using the Scan 4.0 software (NeuroSoft, Inc., El Paso, TX, USA). The artifact-free epochs were averaged separately for each hemisphere to enhance the signal-to-noise ratio. After averaging, all data files were baseline-corrected to the mean of the pre-stimulus window, and digitally low-pass filtered at 50 Hz using a zero-phase shift Butterworth filter (roll-off: 24 dB/octave). For signal analyses involving localization of current sources, the conductive geometry of the head must be approximated with some form of model. Prior studies have indicated using generic spherically symmetric conductor models does not significantly affect localization results or 95% confidence volumes (Teale et al., 2002). Therefore, these methods were used to calculate the overall dimensions of best-fit generic spherical models for each hemisphere, which were used for the localization of neuronal generators. Each spherical model was coregistered with a reconstructed skin-scalp model based on the Montreal-standard MRI volume. For some subjects, anatomical MRIs were available allowing localization results to also be visualized using the actual reconstructed skin-scalp volume.
The procedure for source localization utilized a temporal window between 30-80 ms post-stimulus onset, which was defined uniquely for each hemisphere and subject. Each temporal window included only contiguous data points in which the global field power (GFP) measurement remained at or above 50% of its maximum. The raw GFP is the sum of squares of the magnetic measurements over all sensors for each data point in the averaged epoch, and is calculated independently for each hemisphere and subject. Once this window was identified, the entire sensor array and the Downhill-Simplex optimization algorithm (with five simplex turns) were used to perform a spatiotemporal single-dipole fit on the averaged data for each hemisphere. To be accepted as reliable, the resulting ECD solution had to meet the 0.90 goodness-of-fit (GOF) criterion for all data points within the fit-interval. The fitting interval duration did not differ between hemispheres across the sample, t(32) = 1.04 (P = 0.31), and hemispheric effects as a function of group were also insignificant (see Table 1). The Brain Electrical Source Analysis software (BESA 5.0.6; MEGIS Software GmbH, Germany) was used for all MEG source modeling procedures and GFP calculations.
2.5. Statistical analysis
All statistical analyses used SPSS for Windows (Release 11.0.1). Significance tests were two-tailed and evaluated at 0.05 alpha. Type III sums of squares were used for ANOVA models. Group differences on demographic variables were examined through independent Student’s t-tests. For each MEG source, the coordinates (X, Y, and Z), strength, GOF, and peak-latency were all subjected to independent 2 × 2 mixed-model ANOVA designs, with group as a between-subjects variable and hemisphere as a within-subjects variable. Asymmetry indices were also calculated for direct evaluation of differential source lateralization between groups. For the anterior-posterior (X-coordinate) and superior-inferior (Z-coordinate) planes, the left hemispheric coordinate (i.e., X or Z) was subtracted from that of the right to derive an asymmetry index for each plane per subject. For the medial-lateral plane, the right hemispheric Y-coordinate was added to that of the left (within-subject). Lastly, Pearson-correlation coefficients were used to assess age and inter-hemispheric relationships on source parameters of location, latency, and amplitude.
3. Results
To ensure comparing across different psychoses was justified, preliminary comparisons were performed using all variables and independent t-tests. Results indicated the two psychoses subgroups did not significantly (P > 0.05, uncorrected) differ on any demographic or neural variables, thus collapsing specific diagnoses is reasonable in this group. See Table 1 for descriptive statistics of the psychoses subgroups and controls.
3.1. Source parameters
The main effect of hemisphere for the X-coordinate location (anterior-posterior plane) was significant F(1,31) = 14.79 (P < 0.01), and descriptive statistics indicated left hemispheric sources were more anterior than right hemispheric sources in both groups. However, the group-by-hemisphere interaction term was also significant F(1,31) = 4.25 (P < 0.05), and follow-up t-tests illuminated X-coordinate locations for the right hemisphere were significantly anterior in patients relative to controls, t(31) = 2.11 (P < 0.05), along with no reliable group difference for left hemispheric locations (P > 0.25). To specifically address hemispheric asymmetry, an independent samples t-test was performed using the X-coordinate asymmetry index of each participant, and this measure significantly differed suggesting differential lateralization of sources between groups, t(31) = 2.06 (P < 0.05). In short, a hemispheric asymmetry in the anterior-posterior plane was present only in controls, t(14) = 4.46 (P < 0.001), with the patient data indicating sources were more-or-less symmetrical in this dimension, t(17) = 1.23 (P = 0.24). Figure 1 depicts examples of these asymmetry patterns with localization results from a representative control (A) and patient (C), as well as profile plots (B) of mean X-coordinate locations for each hemisphere per group. As for the Y- and Z-coordinate locations, main effects, interaction effects, and asymmetry indexes were all insignificant. There were also no significant effects for the measures of latency, GOF, or amplitude.
Figure 1.
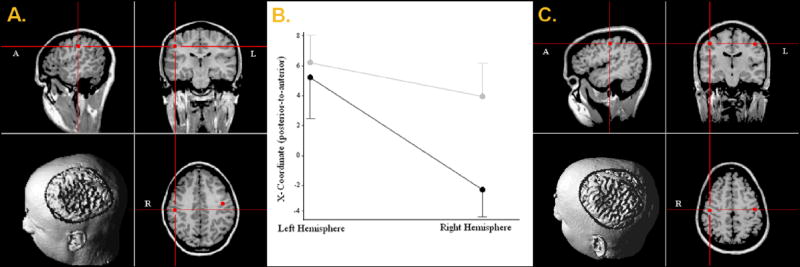
A-C. The anterior-posterior hemispheric asymmetry observed in control subjects (A), the statistically symmetrical pattern detected in patients (C), and a profile plot of X-coordinate locations for each group (B). In (A) and (C), source localization results for both hemispheres are depicted on the respective participant’s MRI volume, with 2D-images and 3D-renditions displayed in radiological convention (note labels in each thumbnail). In (B), the cerebral hemispheres are along the abscissa with the ordinate showing mean X-coordinate values (in mm) for each group. Gray lines represent the early-onset psychosis group and black lines depict control group data. Error bars indicate one standard error of the mean. As shown, left-hemispheric sources were reliably anterior to those of the right only in adolescent controls.
To assess age-related developmental trends, Pearson-correlation coefficients were computed for each group using subject age and X-coordinate source location per hemisphere. Results indicated a trend-level effect for the left hemisphere in controls, with locations tending to be more posterior in older adolescents, r(15) = -0.36 (P < 0.10). In contrast, there was no distinguishable relationship between age and right hemisphere X-coordinate in controls (r(15) = -0.11 (P > 0.25)), or any association between age and either hemisphere in patients (left: r(18) = 0.11 (P > 0.25); right: r(18) = 0.14 (P > 0.25)). In sum, the only developmental marker for lateralization was a posterior shift for sources in the left hemisphere, and this trend was only detected in normal adolescents.
Lastly, the coordination of right- and left-hemispheric developmental processes was assessed by computing Pearson-correlation coefficients for the two hemispheres using peak latency and source amplitude. In controls, results indicated strong positive correlations for peak latency, r(15) = 0.588 (P < 0.01), and source amplitude, r(15) = 0.41 (P < 0.05). Conversely, neither peak latency, r(18) = 0.04 (P > 0.25), or source amplitude, r(18) = 0.13 (P > 0.25)), showed an inter-hemispheric association in patients, which suggests the cerebral hemispheres develop more-or-less along parallel paths only in adolescent controls.
4. Discussion
This study investigated the spatiotemporal dynamics of somatosensory cortical responses in a group of healthy adolescents, and a matched-sample with psychosis. Results showed the latency of these cortical responses were similar to adult findings (Reite et al., 2003), and that latency does not distinguish adolescent patients from controls. Conversely, the localization results suggested significant departure from observations in healthy adults (Reite et al., 1999a, 1999b, 2003), as adolescent controls displayed a reversed pattern of left anterior to right hemispheric asymmetry. Meanwhile, in adolescent patients, the localization finding of statistical symmetry in the axial plane agreed with previous reports indicating adults with neurodevelopmental disorders exhibit either revered hemispheric asymmetry, or an overall lack of hemispheric asymmetry in modality-specific cortices (Reite et al., 1989, 1997, 1999a, 1999b, 2003; Tiihonen et al., 1998; Teale et al., 2000, 2003; Rockstroh et al., 2001; Rojas et al., 2001, 2002; Heim et al., 2003a, 2004). Below, the implications of these findings for the potential specificity of abnormal somatosensory asymmetries are discussed, with especial regard to maturational processes of the region in both health and disease.
This is the first report to our knowledge on somatosensory organization during adolescence, and the results suggest that the functional organization of this region, like the auditory cortex, differs with mental health status and possibly with age. Considering previous adult findings (Reite et al., 2003), the reversed pattern of functional asymmetry in healthy adolescents was contrary to expectations. Reite et al. (2003) utilized the same MEG instrument, recording parameters, experimental stimuli, and presentation procedures as the present study. Therefore, data between these studies should have been closely comparable, and a replication of the adult findings was predicted. For the left hemisphere, this prediction was mostly accurate as the mean anterior-posterior coordinate in these adolescents was only 3 mm anterior from that observed in healthy adults (Reite et al., 2003). This difference is within the error range of MEG source localization and is easily attributable to the random noise inherent in all functional brain measurements. Furthermore, a trend suggesting left-hemispheric generators moving more posterior with age was detected in healthy adolescents, which would be expected given the adult data and could indicate these adolescents will ultimately exhibit sources more posterior then those observed in comparable adults (Reite et al., 2003). Mean M50 generators of the right hemisphere were much less congruent, however, being almost a full centimeter posterior in healthy adolescents compared to their adult counterparts (Reite et al., 2003). These results could reflect a temporal lag in maturation of the non-dominant hemisphere (see Gage et al., 2003; Gogtay et al., 2004), and older adolescents may have shown more adult-like locations or the emergence of a source location and age correlation.
By comparison, the early-onset psychosis group exhibited a pattern of functional hemispheric asymmetry largely matching that predicted, being statistically symmetrical in the anterior-posterior plane. This finding corroborates many previous studies demonstrating altered patterns of hemispheric symmetry or asymmetry in auditory (Reite et al., 1989, 1997; Hajek et al., 1997a; Tiihonen et al., 1998; Teale et al., 2000, 2003; Rockstroh et al., 2001; Rojas et al., 2001, 2002; Heim et al., 2003a, 2004) and somatosensory regions (Reite et al., 1999a, 1999b, 2003) of patients diagnosed with various neurodevelopmental disorders. Interestingly, such aberrations in somatosensory cortices may show greater diagnostic specificity than those observed in auditory areas. As mentioned in the introduction, current data suggests abnormalities in auditory organization are found across a host of different disorders (e.g., developmental dyslexia, fragile X syndrome, and others), whereas those associated with somatosensory regions are only observed in patients with psychotic disorders. In fact, even in patients with the same basic diagnosis (i.e., bipolar I disorder), Reite et al. (1999a) demonstrated only those with psychosis exhibited anomalous asymmetry in this neural region, as patients without psychotic features showed normal right anterior to left asymmetry in somatosensory cortices. Thus, the current results extend these data by establishing abnormal somatosensory asymmetry is present in adolescents with psychosis, and also support the notion that this measure may be useful as a biological marker for psychosis. However, before this asymmetry index can be considered a sensible marker, negative findings in patients with other psychiatric illnesses will need to be reported, as it is currently unclear whether somatosensory asymmetries have been as widely examined as those of the auditory region. Longitudinal studies will also be necessary to map the developmental course of this potential marker. Such studies are especially important given the current findings in young controls, as well as the recent structural data indicating extensive cortical and ventricular changes during adolescence (Frazier et al., 1996; Rapoport et al., 1997; James et al., 1999; Thompson et al., 2001; Gogtay et al., 2004; Sowell et al., 2004). Potentially, such structural changes could differentially modulate asymmetry indexes in cases of normal versus aberrant neurodevelopment.
A variety of behavioral measures have shown a reduction in cerebral laterality is strongly associated with psychotic and non-psychotic neurodevelopmental disorders (e.g., Gur, 1977; Richardson, 1994; Crow et al., 1996; Mitchell and Crow, 2005). However, to date, the genes involved, their active time courses and detrimental effects on observable neurobiology (if any) remain grossly underspecified. Several normative studies have suggested cortical development follows a progressive cycle that largely unfolds during late childhood and throughout adolescence (Sowell et al., 2003, 2004; Giedd et al., 2004; Gogtay et al., 2004). This cycle entails a prominent focus on maturation in the dominant hemisphere, which later expands to homologue areas of the non-dominant hemisphere. The only comparable data involving abnormal neurodevelopment showed accelerated right-hemispheric maturation during late childhood, with cortical development becoming more bilaterally equivalent as the group with psychosis approached young adulthood (Thompson et al., 2001). Thus, in these patients, the normal sequential progression of hemispheric maturation was seemingly disturbed. Results indicating aberrant functional hemispheric asymmetries in adolescent and adult psychosis could therefore be a corollary of subtle structural differences in cortical circuitry. If so, this would at least partially explain why only adolescent controls exhibited strong inter-hemispheric correlations on the source parameters of amplitude and latency. These correlations likely indicate that in normal development, systematic relations exist in the volume (i.e. source amplitude) and latency of somatosensory responses amongst the cerebral hemispheres. Potentially, these inter-hemispheric correlations reflect bilateral coordination of cortical maturation in sensorimotor cortices. Conversely, a lack of such correlations, as exhibited in the early-onset psychosis group, may indicate the cerebral hemispheres are developing along more independent trajectories. It is possible that such coordination problems are associated with the prominent sensorimotor deficits observed during soft neurological examinations in adults and adolescents with psychotic illnesses (Heinrichs and Buchanan, 1988; Karp et al., 2001; Negash et al., 2004), but this speculation will need to be directly examined by comparing neurobehavioral data to the functional imaging results of future studies.
Prior studies have shown later latencies for auditory cortical responses in healthy adolescence relative to adulthood (Rojas et al., 1998; Gage et al., 2003), and similar although truncated results were expected for somatosensory cortices due to earlier structural maturation in this region (Sowell et al., 2003; Giedd, 2004). Thus, the age-mediated latency effects of auditory cortex were expected to be reduced or absent in somatosensory cortices, and this was supported by the current observations. Lastly, in agreement with adult studies (Reite et al., 1999a, 1999b, 2003), the latency of adolescent somatosensory responses did not exhibit a significant relationship to psychosis.
Finally, it is important to recognize some of the limitations of the present work. First, the early-onset psychosis patients were all medicated and the possible influence of medication effects cannot be definitely determined from available data. It is well-appreciated that antipsychotic and other psychoactive agents can influence the frequency and amplitude composition of both spontaneous and evoked EEG and MEG activity (Roemer and Shagass, 1990; Czobor and Volavka, 1993; Canive et al., 1998; Sperling et al., 1999; Rosburg et al., 2000; Centorrino et al., 2002b). Although these signal parameters can be affected, especially for long-latency components, there is currently no evidence that early somatosensory responses can be influenced by medication in a way that could account for the present results. In addition, estimated source strength differences were not detected between groups, which should be the variable most sensitive to medication effects.
In conclusion, this study demonstrated reduced inter-hemispheric developmental coordination, as well as outright alteration in the functional organization of primary-somatosensory cortex in adolescents diagnosed with early-onset psychosis. These observations suggest aberrant cortical maturation processes contribute to the reduction in cerebral laterality commonly associated with psychosis, and provide new evidence for inter-hemispheric interaction accounts of the disorder (Crow et al., 1996).
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute of Mental Health (R01 MH63442-02), and the Developmental Psychobiology Research Group (DPRG) of the University of Colorado Health Sciences Center, Denver, CO, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Annett M. Left, Right, Hand and Brain: The Right Shift Theory. Lawrence Erlbaum Associates; Hillsdale, NJ: 1985. [Google Scholar]
- Canive JM, Lewine JD, Edgar JC, Davis JT, Miller GA, Torres F, Tuason VB. Spontaneous brain magnetic activity in schizophrenia patients treated with aripiprazole. Psychopharmacology Bulletin. 1998;34:101–105. [PubMed] [Google Scholar]
- Centorrino F, Eakin M, Bahk WM, Kelleher JP, Goren J, Salvatore P, Egli S, Baldessarini RJ. Inpatient antipsychotic drug use in 1998, 1993, and 1989. American Journal of Psychiatry. 2002a;159:1932–1935. doi: 10.1176/appi.ajp.159.11.1932. [DOI] [PubMed] [Google Scholar]
- Centorrino F, Price BH, Tuttle M, Bahk WM, Hennen J, Albert MJ, Baldessarini RJ. EEG abnormalities during treatment with typical and atypical antipsychotics. American Journal of Psychiatry. 2002b;159:109–115. doi: 10.1176/appi.ajp.159.1.109. [DOI] [PubMed] [Google Scholar]
- Crognale MA, Page JW, Fuhrel A. Aging of the chromatic onset visual evoked potential. Optometry and Vision Science. 2001;78:442–446. doi: 10.1097/00006324-200106000-00018. [DOI] [PubMed] [Google Scholar]
- Crow TJ, Done DJ, Sacker A. Cerebral lateralization is delayed in children who later develop schizophrenia. Schizophrenia Research. 1996;22:181–185. doi: 10.1016/s0920-9964(96)00068-0. [DOI] [PubMed] [Google Scholar]
- Czobor P, Volavka J. Quantitative electroencephalogram examination of effects of risperidone in schizophrenic patients. Journal of Clinical Psychopharmacology. 1993;13:332–342. [PubMed] [Google Scholar]
- Frazier JA, Giedd JN, Hamburger SD, Albus KE, Kaysen D, Vaituzis AC, Rajapakse JC, Lenane MC, McKenna K, Jacobsen LK, Gordon CT, Breier A, Rapoport JL. Brain anatomic magnetic resonance imaging in childhood-onset schizophrenia. Archives of General Psychiatry. 1996;53:617–624. doi: 10.1001/archpsyc.1996.01830070065010. [DOI] [PubMed] [Google Scholar]
- Gage NM, Siegel B, Roberts TPL. Cortical auditory system maturational abnormalities in children with autism disorder: An MEG investigation. Developmental Brain Research. 2003;144:201–209. doi: 10.1016/s0165-3806(03)00172-x. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Gledd JN, Lusk L, Hayashi KM, Greenstein D, Valtuzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE. Motoric laterality imbalance in schizophrenia. Archives of General Psychiatry. 1977;34:33–37. doi: 10.1001/archpsyc.1977.01770130035003. [DOI] [PubMed] [Google Scholar]
- Hajek M, Boehle C, Huonker R, Volz HP, Nowak H, Schrott PR, Sauer H. Abnormalities of auditory evoked magnetic fields in the right hemisphere of schizophrenic females. Schizophrenia Research. 1997a;24:329–332. doi: 10.1016/s0920-9964(96)00107-7. [DOI] [PubMed] [Google Scholar]
- Hajek M, Huonker R, Boehle C, Volz HP, Nowak H, Sauer H. Abnormalities of auditory evoked magnetic fields and structural changes in the left hemisphere of male schizophrenics: A magnetoencephalographic-magnetic resonance imaging study. Biological Psychiatry. 1997b;42:609–616. doi: 10.1016/s0006-3223(96)00428-3. [DOI] [PubMed] [Google Scholar]
- Heim S, Eulitz C, Elbert T. Altered hemispheric asymmetry of auditory N100m in adults with developmental dyslexia. NeuroReport. 2003a;14:501–504. doi: 10.1097/00001756-200303030-00041. [DOI] [PubMed] [Google Scholar]
- Heim S, Eulitz C, Elbert T. Altered hemispheric asymmetry of auditory P100m in dyslexia. European Journal of Neuroscience. 2003b;17:1715–1722. doi: 10.1046/j.1460-9568.2003.02596.x. [DOI] [PubMed] [Google Scholar]
- Heim S, Kissler J, Elbert T, Rockstroh B. Cerebral lateralization in schizophrenia and dyslexia: Neuromagnetic responses to auditory stimuli. Neuropsychologia. 2004;42:692–697. doi: 10.1016/j.neuropsychologia.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Heinrichs DW, Buchanen RW. Significance and meaning of neurological signs in schizophrenia. American Journal of Psychiatry. 1988;145:11–18. doi: 10.1176/ajp.145.1.11. [DOI] [PubMed] [Google Scholar]
- Hoechstetter K, Rupp A, Stancak A, Meinck HM, Stippich C, Berg P, Scherg M. Interaction of tactile input in the human primary and secondary somatosensory cortex — A magnetoencephalographic study. Neuroimage. 2001;14:759–767. doi: 10.1006/nimg.2001.0855. [DOI] [PubMed] [Google Scholar]
- Iguchi Y, Hoshi Y, Hashimoto I. Selective spatial attention induces short-term plasticity in human somatosensory cortex. Neuroreport. 2001;12:3133–3136. doi: 10.1097/00001756-200110080-00030. [DOI] [PubMed] [Google Scholar]
- Isohanni M, Jones PB, Moilanen K, Rantakallio P, Veijola J, Oja H, Koiranen M, Jokelainen J, Croudace T, Jarvelin M. Early developmental milestones in adult schizophrenia and other psychoses. A 31-year follow-up of the northern Finland 1966 birth cohort. Schizophrenia Research. 2001;52:1–19. doi: 10.1016/s0920-9964(00)00179-1. [DOI] [PubMed] [Google Scholar]
- James ACD, Crow TJ, Renowden S, Wardell AMJ, Smith DM, Anslow P. Is the course of brain development in schizophrenia delayed? Evidence from onsets in adolescence. Schizophrenia Research. 1999;40:1–10. doi: 10.1016/s0920-9964(99)00042-0. [DOI] [PubMed] [Google Scholar]
- Kakigi R, Hoshiyama M, Shimojo M, Naka D, Yamasaki H, Watanabe S, Xiang J, Maeda K, Lam K, Itomi K, Nakamura A. The Somatosensory evoked magnetic fields. Progress in Neurobiology. 2000;61:495–523. doi: 10.1016/s0301-0082(99)00063-5. [DOI] [PubMed] [Google Scholar]
- Karp BI, Garvey M, Jacobsen LK, Frazier JA, Hamburger SD, Bedwell JS, Rapoport JL. Abnormal neurologic maturation in adolescents with early-onset schizophrenia. American Journal of Psychiatry. 2001;158:118–122. doi: 10.1176/appi.ajp.158.1.118. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for affective disorders and schizophrenia for school-age children – present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Mitchell RLC, Crow TJ. Right hemisphere language functions and schizophrenia: The forgotten hemisphere? Brain. 2005;128:963–978. doi: 10.1093/brain/awh466. [DOI] [PubMed] [Google Scholar]
- Negash A, Kebede D, Alem A, Melaku Z, Deyessa N, Shibire T, Fekadu A, Fekadu D, Jacobsson L, Kullgren G. Neurological soft signs in bipolar I disorder patients. Journal of Affective Disorders. 2004;80:221–230. doi: 10.1016/S0165-0327(03)00116-2. [DOI] [PubMed] [Google Scholar]
- Paul I, Bott C, Heim S, Eulitz C, Elbert T. Reduced hemispheric asymmetry of the auditory N260m in dyslexia. Neuropsychologia. 2006;44:785–794. doi: 10.1016/j.neuropsychologia.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Giedd JN, Kumra S, Jacobsen L, Smith A, Lee P, Nelson J, Hamburger S. Childhood-onset schizophrenia: Progressive ventricular change during adolescence. Archives of General Psychiatry. 1997;54:897–903. doi: 10.1001/archpsyc.1997.01830220013002. [DOI] [PubMed] [Google Scholar]
- Reite M, Sheeder J, Teale P, Adams M, Richardson D, Simon J, Jones RH, Rojas DC. Magnetic source imaging evidence of sex differences in cerebral lateralization in schizophrenia. Archives of General Psychiatry. 1997;54:433–440. doi: 10.1001/archpsyc.1997.01830170059009. [DOI] [PubMed] [Google Scholar]
- Reite M, Teale P, Goldstein L, Whalen J, Linnville S. Late auditory sources may differ in the left hemisphere of schizophrenic patients: A preliminary report. Archives of General Psychiatry. 1989;46:565–572. doi: 10.1001/archpsyc.1989.01810060087013. [DOI] [PubMed] [Google Scholar]
- Reite M, Teale P, Rojas DC, Arciniegas D, Sheeder J. Bipolar disorder: Anomalous brain asymmetry associated with psychosis. American Journal of Psychiatry. 1999a;156:1159–1163. doi: 10.1176/ajp.156.8.1159. [DOI] [PubMed] [Google Scholar]
- Reite M, Teale P, Rojas DC, Benkers TL, Carlson J. Anomalous somatosensory cortical localization in schizophrenia. American Journal of Psychiatry. 2003;160:2148–2153. doi: 10.1176/appi.ajp.160.12.2148. [DOI] [PubMed] [Google Scholar]
- Reite M, Teale P, Rojas DC, Sheeder J, Arciniegas D. Schizoaffective disorder: Evidence for reversed cerebral asymmetry. Biological Psychiatry. 1999b;46:133–136. doi: 10.1016/s0006-3223(99)00053-0. [DOI] [PubMed] [Google Scholar]
- Richardson AJ. Dyslexia, handedness and syndromes of psychosis-proneness. International Journal of Psychophysiology. 1994;18:251–263. doi: 10.1016/0167-8760(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Rockstroh B, Kissler J, Mohr B, Eulitz C, Lommen U, Wienbruch C, Cohen R, Elbert T. Altered hemispheric asymmetry of auditory magnetic fields to tones and syllables in schizophrenia. Biological Psychiatry. 2001;49:694–703. doi: 10.1016/s0006-3223(00)01023-4. [DOI] [PubMed] [Google Scholar]
- Roemer RA, Shagass C. Replication of an evoked potential study of lateralized hemispheric dysfunction in schizophrenics. Biological Psychiatry. 1990;28:275–291. doi: 10.1016/0006-3223(90)90655-l. [DOI] [PubMed] [Google Scholar]
- Rojas DC, Bawn SD, Carlson JP, Arciniegas DB, Teale PD, Reite ML. Alterations in tonotopy and auditory cerebral asymmetry in schizophrenia. Biological Psychiatry. 2002;52:32–39. doi: 10.1016/s0006-3223(01)01365-8. [DOI] [PubMed] [Google Scholar]
- Rojas DC, Benkers TL, Rogers SJ, Teale PD, Reite ML, Hagerman RJ. Auditory evoked magnetic fields in adults with fragile X syndrome. NeuroReport. 2001;12:2573–2576. doi: 10.1097/00001756-200108080-00056. [DOI] [PubMed] [Google Scholar]
- Rojas DC, Teale PD, Sheeder JL, Simon J, Reite ML. Sex-specific expression of heschl’s gyrus functional and structural abnormalities in paranoid schizophrenia. American Journal of Psychiatry. 1997;154:1655–1662. doi: 10.1176/ajp.154.12.1655. [DOI] [PubMed] [Google Scholar]
- Rojas DC, Walker JR, Sheeder JL, Teale PD, Reite ML. Developmental changes in refractoriness of the neuromagnetic M100 in children. NeuroReport. 1998;9:1543–1547. doi: 10.1097/00001756-199805110-00055. [DOI] [PubMed] [Google Scholar]
- Rosburg T, Kreitschmann-Andermahr I, Ugur T, Nestmann H, Nowak H, Sauer H. Tonotopy of the auditory-evoked field component N100m in patients with schizophrenia. International Journal of Psychophysiology. 2000;14:131–141. [Google Scholar]
- Sigurdsson E, Fombonne E, Sayal K, Checkley S. Neurodevelopmental antecedents of early-onset bipolar affective disorder. British Journal of Psychiatry. 1999;174:121–127. doi: 10.1192/bjp.174.2.121. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nature Neuroscience. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. Journal of Neuroscience. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling W, Vieth J, Martus M, Demling J, Barocka A. Spontaneous slow and fast MEG activity in male schizophrenics treated with clozapine. Psychopharmacology. 1999;142:375–382. doi: 10.1007/s002130050902. [DOI] [PubMed] [Google Scholar]
- Teale PD, Benkers T, Rojas DC, Reite ML. Determination of the sphere origin for MEG source modeling in temporal regions. Physics in Medicine and Biology. 2002;47:1161–1166. doi: 10.1088/0031-9155/47/7/311. [DOI] [PubMed] [Google Scholar]
- Teale PD, Carlson JP, Rojas DC, Reite ML. Reduced laterality of the source locations for generators of the auditory steady state field in schizophrenia. Biological Psychiatry. 2003;54:1149–1153. doi: 10.1016/s0006-3223(03)00411-6. [DOI] [PubMed] [Google Scholar]
- Teale PD, Reite ML, Rojas DC, Sheeder JL, Arciniegas DB. Fine structure of the auditory M100 in schizophrenia and schizoaffective disorder. Biological Psychiatry. 2000;48:1109–1112. doi: 10.1016/s0006-3223(00)00941-0. [DOI] [PubMed] [Google Scholar]
- Thatcher RW, Walker RA, Giudice S. Human cerebral hemispheres develop at different rates and ages. Science. 1987;236:1110–1114. doi: 10.1126/science.3576224. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Vidal C, Giedd JN, Gochman P, Blumenthal J, Nicolson R, Toga AW, Rapoport JL. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proceedings of the National Academy of Sciences USA. 2001;98:11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiihonen J, Katila H, Pekkonen E, Jaaskelainen IP, Huotilainen M, Aronen HJ, Ilmoniemi RJ, Rasanen P, Virtanen J, Salli E, Karhu J. Reversal of cerebral asymmetry in schizophrenia measured with magnetoencephalography. Schizophrenia Research. 1998;30:209–219. doi: 10.1016/s0920-9964(97)00154-0. [DOI] [PubMed] [Google Scholar]
- Tonnquist-Uhlen I, Borg E, Spens KE. Topography of auditory-evoked long-latency potential in normal-children, with particular reference to the N1 component. Electroencephalography and Clinical Neurophysiology. 1995;95:34–41. doi: 10.1016/0013-4694(95)00044-y. [DOI] [PubMed] [Google Scholar]
- Wikstrom H, Roine RO, Salonen O, Aronen HJ, Virtanen J, Ilmoniemi RJ, Huttunen J. Somatosensory evoked magnetic fields to median nerve stimulation: Interhemispheric differences in a normal population. Electroencephalography and Clinical Neurophysiology. 1997;104:480–487. doi: 10.1016/s0168-5597(97)00055-5. [DOI] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. The Journal of Clinical Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Yang TT, Gallen CC, Schwartz B, Bloom FE. Noninvasive somatosensory homunculus mapping in humans by using a large-array biomagnetometer. Proceedings of the National Academy of Sciences USA. 1993;90:3098–3102. doi: 10.1073/pnas.90.7.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


