Abstract
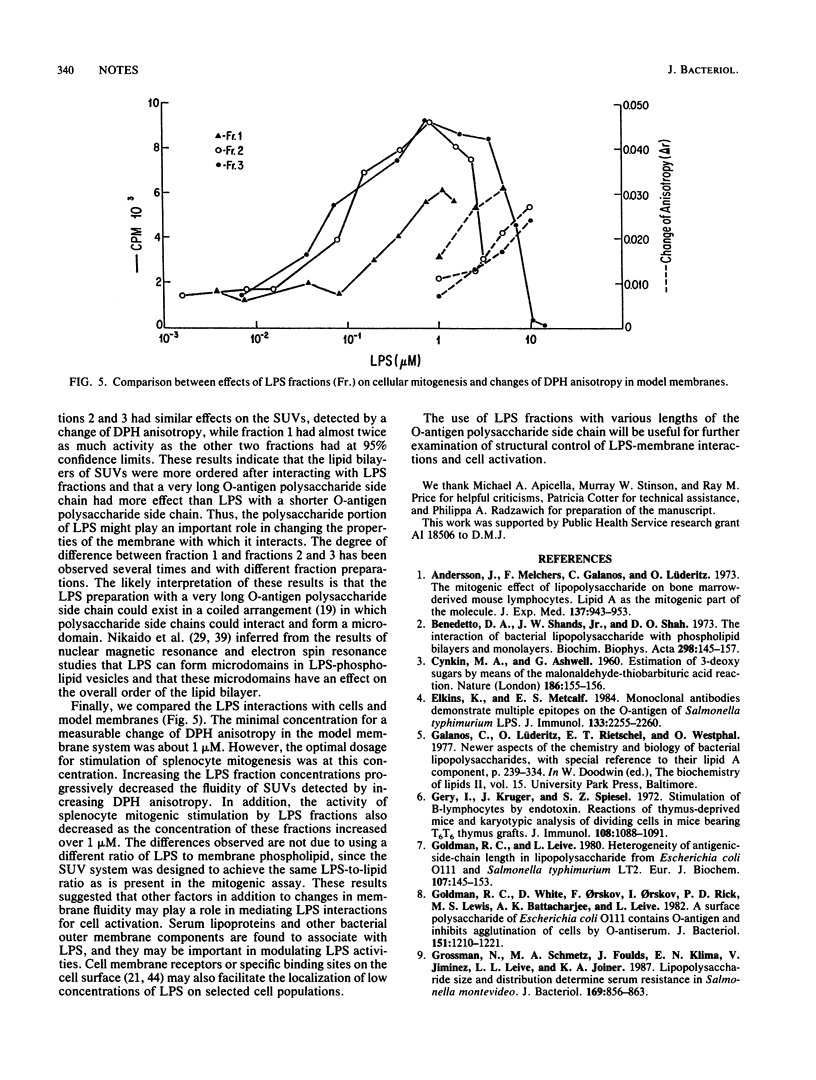
The role of the length of the O-antigen polysaccharide side chain of bacterial lipopolysaccharide (LPS) in biological and model membrane systems was investigated. LPS from Salmonella typhimurium ATCC 14028 was chromatographed on a Sephadex G-200 column in the presence of sodium deoxycholate and separated into three fractions on the basis of molecular size. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis, Western blot (immunoblot), and chemical analyses indicated that these fractions differed from each other primarily in the number of repeating units in the O-antigen polysaccharide side chain. In a biological system fractions 2 and 3 had the same effects to induce mitogenesis in murine lymphocytes, but fraction 1 was less effective than the other two fractions. In a model membrane system, LPS induced changes in small unilamellar vesicles (SUVs) which were measured by changes in the behavior of a fluorescent probe, 1,6-diphenylhexa-1,3,5-triene (DPH), and interaction of increasing amounts of all LPS fractions with SUVs gradually increased DPH anisotropy. Fractions 2 and 3 had similar effects on the SUVs as detected by changes in DPH anisotropy, while fraction 1 had almost twice as much activity as the other two fractions. These results suggest that the polysaccharide side chain of LPS may modulate the ability of biologically active lipid A to interact with cells and model membranes. In addition, factors other than changes in membrane fluidity may play a role in mediating LPS-induced cell activation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Melchers F., Galanos C., Lüderitz O. The mitogenic effect of lipopolysaccharide on bone marrow-derived mouse lymphocytes. Lipid A as the mitogenic part of the molecule. J Exp Med. 1973 Apr 1;137(4):943–953. doi: 10.1084/jem.137.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetto D. A., Shands J. W., Jr, Shah D. O. The interaction of bacterial lipopolysaccharide with phospholipid bilayers and monolayers. Biochim Biophys Acta. 1973 Mar 16;298(2):145–157. doi: 10.1016/0005-2736(73)90346-5. [DOI] [PubMed] [Google Scholar]
- CYNKIN M. A., ASHWELL G. Estimation of 3-deoxy sugars by means of the malonaldehyde-thiobarbituric acid reaction. Nature. 1960 Apr 9;186:155–156. doi: 10.1038/186155a0. [DOI] [PubMed] [Google Scholar]
- Elkins K., Metcalf E. S. Monoclonal antibodies demonstrate multiple epitopes on the O antigens of Salmonella typhimurium LPS. J Immunol. 1984 Oct;133(4):2255–2260. [PubMed] [Google Scholar]
- Gery I., Krüger J., Spiesel S. Z. Stimulation of B-lymphocytes by endotoxin. Reactions of thymus-deprived mice and karyotypic analysis of dividing cells in mice bearing T 6 T 6 thymus grafts. J Immunol. 1972 Apr;108(4):1088–1091. [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Goldman R. C., White D., Orskov F., Orskov I., Rick P. D., Lewis M. S., Bhattacharjee A. K., Leive L. A surface polysaccharide of Escherichia coli O111 contains O-antigen and inhibits agglutination of cells by O-antiserum. J Bacteriol. 1982 Sep;151(3):1210–1221. doi: 10.1128/jb.151.3.1210-1221.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman N., Schmetz M. A., Foulds J., Klima E. N., Jimenez-Lucho V. E., Leive L. L., Joiner K. A., Jiminez V. Lipopolysaccharide size and distribution determine serum resistance in Salmonella montevideo. J Bacteriol. 1987 Feb;169(2):856–863. doi: 10.1128/jb.169.2.856-863.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J. Aberrant migration of lipopolysaccharide in sodium dodecyl sulfate/polyacrylamide gel electrophoresis. Eur J Biochem. 1983 Jul 1;133(3):685–688. doi: 10.1111/j.1432-1033.1983.tb07517.x. [DOI] [PubMed] [Google Scholar]
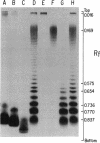
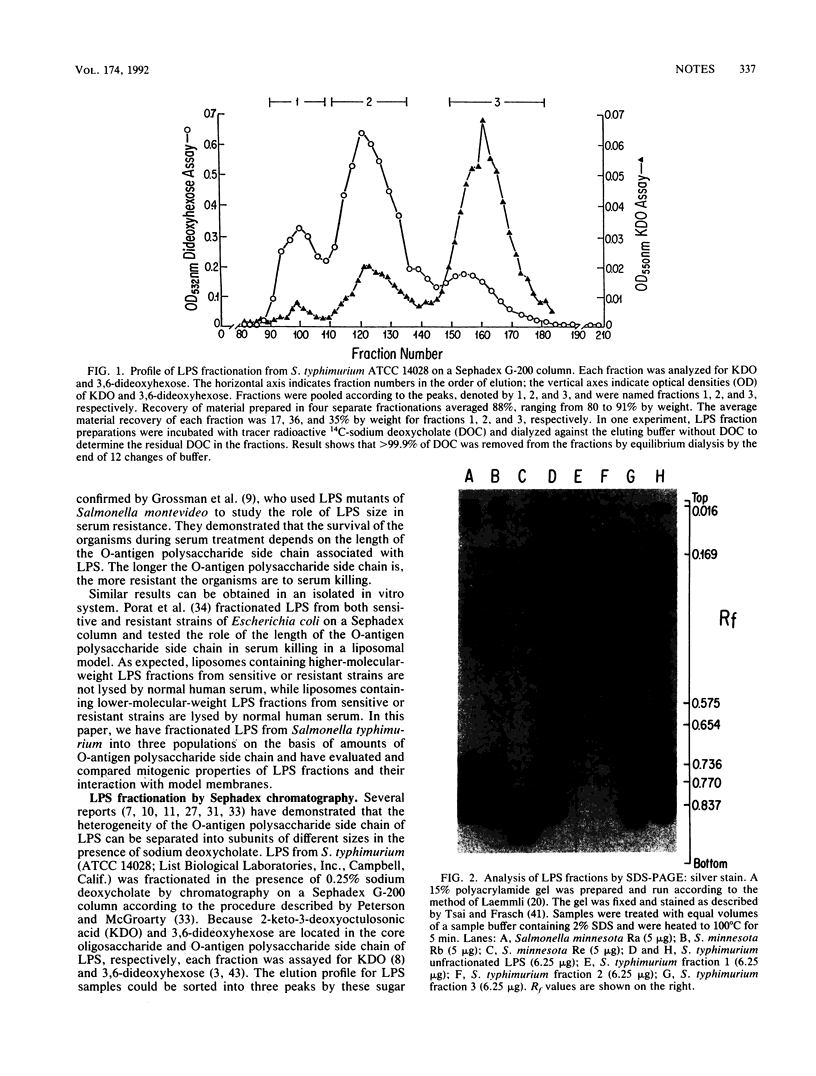
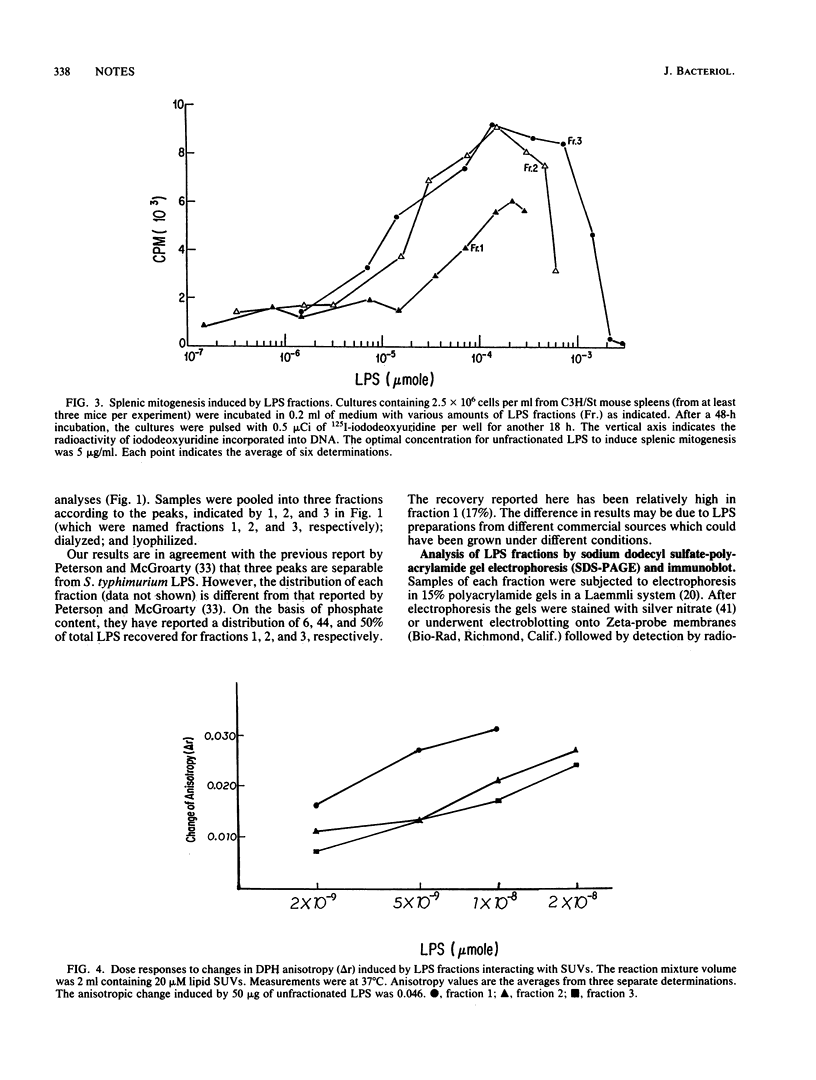
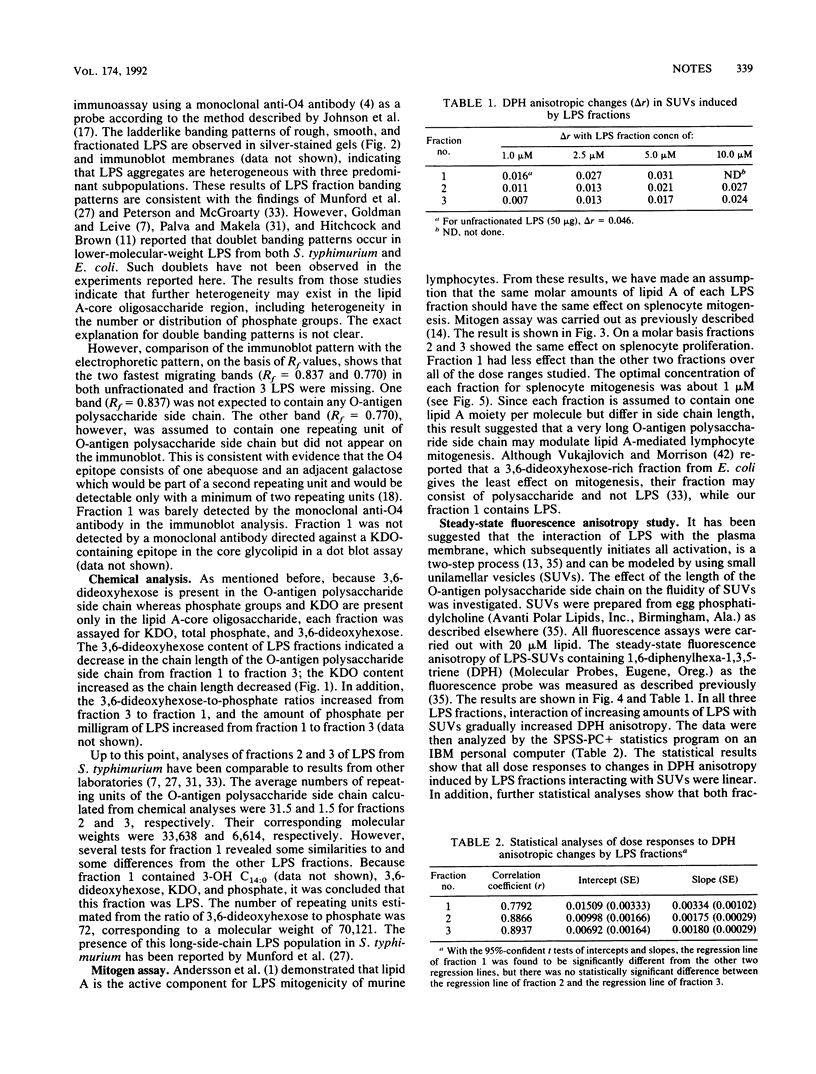
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Leive L., Mäkelä P. H., Rietschel E. T., Strittmatter W., Morrison D. C. Lipopolysaccharide nomenclature--past, present, and future. J Bacteriol. 1986 Jun;166(3):699–705. doi: 10.1128/jb.166.3.699-705.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs D. M. Structural features of binding of lipopolysaccharides to murine lymphocytes. Rev Infect Dis. 1984 Jul-Aug;6(4):501–505. doi: 10.1093/clinids/6.4.501. [DOI] [PubMed] [Google Scholar]
- Jacobs M. D., Morrison D. C. Dissociation between mitogenicity and immunogenicity of TNP-lipopolysaccharide, a T-independent antigen. J Exp Med. 1975 Jun 1;141(6):1453–1458. doi: 10.1084/jem.141.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessop H. L., Lambert P. A. The role of surface polysaccharide in determining the resistance of Serratia marcescens to serum killing. J Gen Microbiol. 1986 Sep;132(9):2505–2514. doi: 10.1099/00221287-132-9-2505. [DOI] [PubMed] [Google Scholar]
- Jörbeck H. J., Svenson S. B., Lindberg A. A. Immunochemistry of Salmonella O-antigens: specificity of rabbit antibodies against the O-antigen 4 determinant elicited by whole bacteria and O-antigen 4 specific saccharide-protein conjugates. J Immunol. 1979 Sep;123(3):1376–1381. [PubMed] [Google Scholar]
- Labischinski H., Barnickel G., Bradaczek H., Naumann D., Rietschel E. T., Giesbrecht P. High state of order of isolated bacterial lipopolysaccharide and its possible contribution to the permeation barrier property of the outer membrane. J Bacteriol. 1985 Apr;162(1):9–20. doi: 10.1128/jb.162.1.9-20.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lei M. G., Morrison D. C. Specific endotoxic lipopolysaccharide-binding proteins on murine splenocytes. I. Detection of lipopolysaccharide-binding sites on splenocytes and splenocyte subpopulations. J Immunol. 1988 Aug 1;141(3):996–1005. [PubMed] [Google Scholar]
- Lüderitz O., Staub A. M., Westphal O. Immunochemistry of O and R antigens of Salmonella and related Enterobacteriaceae. Bacteriol Rev. 1966 Mar;30(1):192–255. doi: 10.1128/br.30.1.192-255.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monner D. A., Jonsson S., Boman H. G. Ampicillin-resistant mutants of Escherichia coli K-12 with lipopolysaccharide alterations affecting mating ability and susceptibility to sex-specific bacteriophages. J Bacteriol. 1971 Aug;107(2):420–432. doi: 10.1128/jb.107.2.420-432.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Bacterial endotoxins and host immune responses. Adv Immunol. 1979;28:293–450. doi: 10.1016/s0065-2776(08)60802-0. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Ulevitch R. J. The effects of bacterial endotoxins on host mediation systems. A review. Am J Pathol. 1978 Nov;93(2):526–618. [PMC free article] [PubMed] [Google Scholar]
- Munford R. S., Hall C. L., Rick P. D. Size heterogeneity of Salmonella typhimurium lipopolysaccharides in outer membranes and culture supernatant membrane fragments. J Bacteriol. 1980 Nov;144(2):630–640. doi: 10.1128/jb.144.2.630-640.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Nakae T. The outer membrane of Gram-negative bacteria. Adv Microb Physiol. 1979;20:163–250. doi: 10.1016/s0065-2911(08)60208-8. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Takeuchi Y., Ohnishi S. I., Nakae T. Outer membrane of Salmonella typhimurium. Electron spin resonance studies. Biochim Biophys Acta. 1977 Feb 14;465(1):152–164. doi: 10.1016/0005-2736(77)90363-7. [DOI] [PubMed] [Google Scholar]
- Onji T., Liu M. S. Changes in surface charge density on liposomes induced by Escherichia coli endotoxin. Biochim Biophys Acta. 1979 Dec 12;558(3):320–324. doi: 10.1016/0005-2736(79)90267-0. [DOI] [PubMed] [Google Scholar]
- Palva E. T., Mäkelä P. H. Lipopolysaccharide heterogeneity in Salmonella typhimurium analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Eur J Biochem. 1980;107(1):137–143. doi: 10.1111/j.1432-1033.1980.tb04634.x. [DOI] [PubMed] [Google Scholar]
- Peavy D. L., Adler W. H., Smith R. T. The mitogenic effects of endotoxin and staphylococcal enterotoxin B on mouse spleen cells and human peripheral lymphocytes. J Immunol. 1970 Dec;105(6):1453–1458. [PubMed] [Google Scholar]
- Peterson A. A., McGroarty E. J. High-molecular-weight components in lipopolysaccharides of Salmonella typhimurium, Salmonella minnesota, and Escherichia coli. J Bacteriol. 1985 May;162(2):738–745. doi: 10.1128/jb.162.2.738-745.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porat R., Johns M. A., McCabe W. R. Selective pressures and lipopolysaccharide subunits as determinants of resistance of clinical isolates of gram-negative bacilli to human serum. Infect Immun. 1987 Feb;55(2):320–328. doi: 10.1128/iai.55.2.320-328.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R. M., Jacobs D. M. Fluorescent detection of lipopolysaccharide interactions with model membranes. Biochim Biophys Acta. 1986 Jul 10;859(1):26–32. doi: 10.1016/0005-2736(86)90314-7. [DOI] [PubMed] [Google Scholar]
- Takano T., Mizuno D. Dynamic state of the spleen cells of mice after administration of the endotoxin of Proteus vulgaris. I. Cellular proliferation after administration of the endotoxin. Jpn J Exp Med. 1968 Jun;38(3):171–183. [PubMed] [Google Scholar]
- Takeuchi Y., Nikaido H. Persistence of segregated phospholipid domains in phospholipid--lipopolysaccharide mixed bilayers: studies with spin-labeled phospholipids. Biochemistry. 1981 Feb 3;20(3):523–529. doi: 10.1021/bi00506a013. [DOI] [PubMed] [Google Scholar]
- Tamaki S., Matsuhashi M. Increase in sensitivity to antibiotics and lysozyme on deletion of lipopolysaccharides in Escherichia coli strains. J Bacteriol. 1973 Apr;114(1):453–454. doi: 10.1128/jb.114.1.453-454.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Vukajlovich S. W., Morrison D. C. Conversion of lipopolysaccharides to molecular aggregates with reduced subunit heterogeneity: demonstration of LPS-responsiveness in "endotoxin-unresponsive" C3H/HeJ splenocytes. J Immunol. 1983 Jun;130(6):2804–2808. [PubMed] [Google Scholar]
- WARAVDEKAR V. S., SASLAW L. D. A sensitive colorimetric method for the estimation of 2-deoxy sugars with the use of the malonaldehyde-thiobarbituric acid reaction. J Biol Chem. 1959 Aug;234(8):1945–1950. [PubMed] [Google Scholar]
- Wollenweber H. W., Morrison D. C. Synthesis and biochemical characterization of a photoactivatable, iodinatable, cleavable bacterial lipopolysaccharide derivative. J Biol Chem. 1985 Dec 5;260(28):15068–15074. [PubMed] [Google Scholar]