Abstract
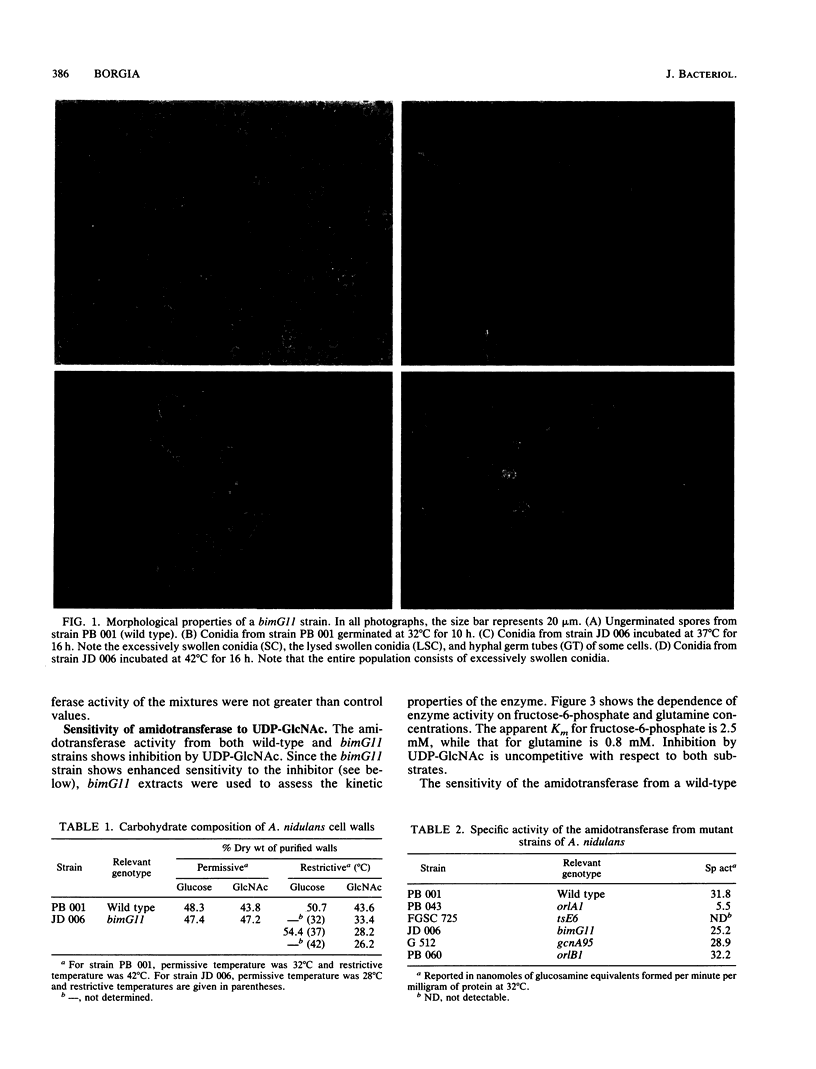
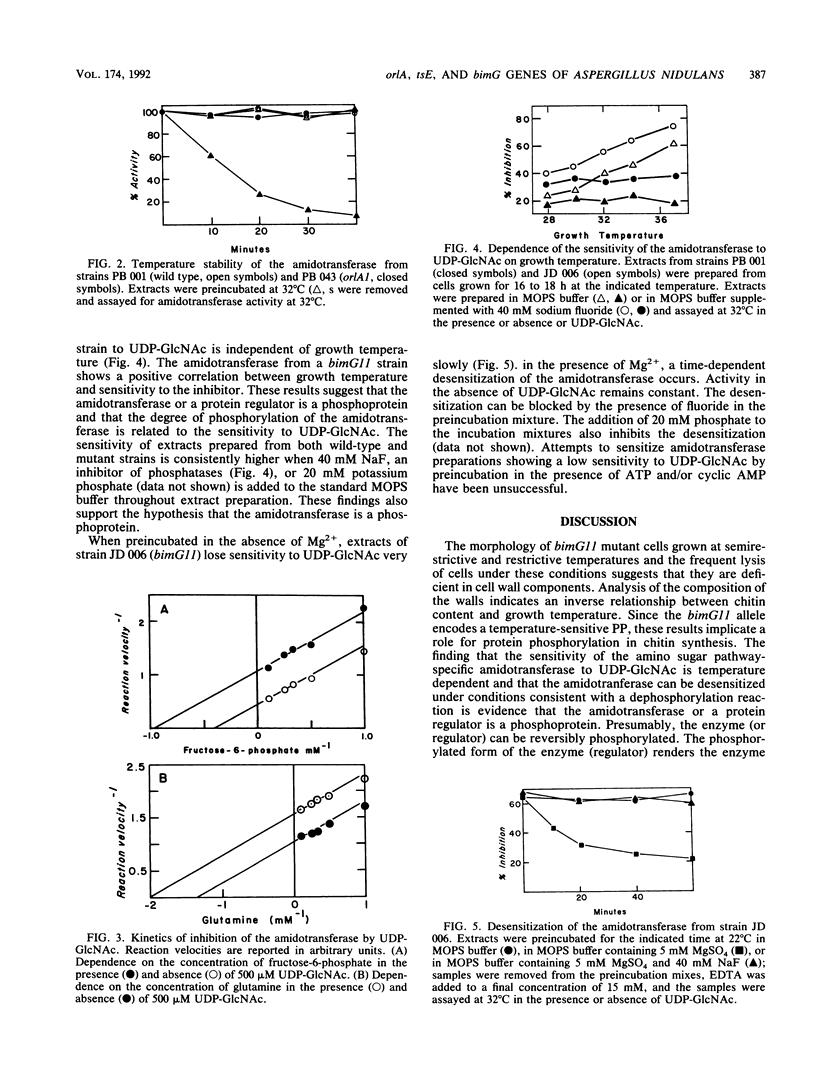
Strains of Aspergillus nidulans carrying the orlA1 or tse6 allele are deficient in cell wall chitin and undergo lysis at restrictive temperatures. The strains are remediable by osmotic stabilizers or by the presence of N-acetylglucosamine (GlcNAc) in the medium. The remediation by GlcNAc suggests that the lesion(s) in chitin synthesis resides in the amino sugar biosynthetic pathway prior to the synthesis of N-acetylglucosamine-6-phosphate. orlA1 strains grown at permissive temperature exhibit an abnormally low specific activity for L-glutamine:fructose-6-phosphate amidotransferase (EC 2.6.1.16, amidotransferase), the first enzyme unique to amino sugar synthesis. In addition, the enzyme produced is temperature sensitive in vitro. tsE6 strains grown at permissive temperature show virtually no amidotransferase activity. This finding is consistent with an extremely labile enzyme which is destroyed by cell breakage and extract preparation. The enzyme must be active in vivo at permissive temperatures since GlcNAc is not required for growth. Thus, two structural genes (orlA and tsE) are necessary for the amidotransferase activity. bimG11 strains are temperature sensitive for a type 1 protein phosphatase involved in cell cycle regulation and arrest in mitosis. Like orlA1 and tsE6 strains, conidia from bimG11 strains swell excessively when germinated and lyse; the germlings produced are deficient in chitin content. The amidotransferase from wild-type and mutant strains is sensitive to feedback inhibition by uridine diphosphate-N-acetylglucosamine. The sensitivity of the amidotransferase from bimG11 strains is dependent on growth temperature, while that from wild-type strains is independent of temperature. The enzyme can be desensitized in vitro under conditions consistent with a protein phosphatase reaction. It is proposed that amino sugar (and chitin biosynthesis) is partially regulated by phosphorylation-dephosphorylation of the amidotransferase or a protein regulator of the enzyme.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartnicki-Garcia S., Lippman E. Fungal morphogenesis: cell wall construction in Mucor rouxii. Science. 1969 Jul 18;165(3890):302–304. doi: 10.1126/science.165.3890.302. [DOI] [PubMed] [Google Scholar]
- Borgia P. T., Dodge C. L. Characterization of Aspergillus nidulans mutants deficient in cell wall chitin or glucan. J Bacteriol. 1992 Jan;174(2):377–383. doi: 10.1128/jb.174.2.377-383.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib E., Bowers B. Chitin and yeast budding. Localization of chitin in yeast bud scars. J Biol Chem. 1971 Jan 10;246(1):152–159. [PubMed] [Google Scholar]
- Cabib E., Roberts R., Bowers B. Synthesis of the yeast cell wall and its regulation. Annu Rev Biochem. 1982;51:763–793. doi: 10.1146/annurev.bi.51.070182.003555. [DOI] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Cyert M. S., Thorner J. Putting it on and taking it off: phosphoprotein phosphatase involvement in cell cycle regulation. Cell. 1989 Jun 16;57(6):891–893. doi: 10.1016/0092-8674(89)90325-5. [DOI] [PubMed] [Google Scholar]
- Domek D. B., Borgia P. T. Changes in the rate of chitin-plus-chitosan synthesis accompany morphogenesis of Mucor racemosus. J Bacteriol. 1981 Jun;146(3):945–951. doi: 10.1128/jb.146.3.945-951.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doonan J. H., Morris N. R. The bimG gene of Aspergillus nidulans, required for completion of anaphase, encodes a homolog of mammalian phosphoprotein phosphatase 1. Cell. 1989 Jun 16;57(6):987–996. doi: 10.1016/0092-8674(89)90337-1. [DOI] [PubMed] [Google Scholar]
- Frisa P. S., Sonneborn D. R. Developmentally regulated interconversions between end product-inhibitable and noninhibitable forms of a first pathway-specific enzyme activity can be mimicked in vitro by protein dephosphorylation-phosphorylation reactions. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6289–6293. doi: 10.1073/pnas.79.20.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy A. M., Zolnierowicz S., Stapleton A. E., Goebl M., DePaoli-Roach A. A., Pringle J. R. CDC55, a Saccharomyces cerevisiae gene involved in cellular morphogenesis: identification, characterization, and homology to the B subunit of mammalian type 2A protein phosphatase. Mol Cell Biol. 1991 Nov;11(11):5767–5780. doi: 10.1128/mcb.11.11.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz D., Rosenberger R. F. A mutation in Aspergillus nidulans producing hyphal walls which lack chitin. Biochim Biophys Acta. 1970 Jun;208(3):452–460. doi: 10.1016/0304-4165(70)90218-7. [DOI] [PubMed] [Google Scholar]
- Katz D., Rosenberger R. F. Hyphal wall synthesis in Aspergillus nidulans: effect of protein synthesis inhibition and osmotic shock on chitin insertion and morphogenesis. J Bacteriol. 1971 Oct;108(1):184–190. doi: 10.1128/jb.108.1.184-190.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita N., Ohkura H., Yanagida M. Distinct, essential roles of type 1 and 2A protein phosphatases in the control of the fission yeast cell division cycle. Cell. 1990 Oct 19;63(2):405–415. doi: 10.1016/0092-8674(90)90173-c. [DOI] [PubMed] [Google Scholar]
- Marchant R., Smith D. G. A serological investigation of hyphal growth in Fusarium culmorum. Arch Mikrobiol. 1968;63(1):85–94. doi: 10.1007/BF00407067. [DOI] [PubMed] [Google Scholar]
- Mäntsälä P., Zalkin H. Glutamine nucleotide sequence of Saccharomyces cerevisiae ADE4 encoding phosphoribosylpyrophosphate amidotransferase. J Biol Chem. 1984 Jul 10;259(13):8478–8484. [PubMed] [Google Scholar]
- Ohkura H., Kinoshita N., Miyatani S., Toda T., Yanagida M. The fission yeast dis2+ gene required for chromosome disjoining encodes one of two putative type 1 protein phosphatases. Cell. 1989 Jun 16;57(6):997–1007. doi: 10.1016/0092-8674(89)90338-3. [DOI] [PubMed] [Google Scholar]
- Orlean P., Arnold E., Tanner W. Apparent inhibition of glycoprotein synthesis by S.cerevisiae mating pheromones. FEBS Lett. 1985 May 20;184(2):313–317. doi: 10.1016/0014-5793(85)80629-3. [DOI] [PubMed] [Google Scholar]
- Read S. M., Northcote D. H. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal Biochem. 1981 Sep 1;116(1):53–64. doi: 10.1016/0003-2697(81)90321-3. [DOI] [PubMed] [Google Scholar]
- Ronne H., Carlberg M., Hu G. Z., Nehlin J. O. Protein phosphatase 2A in Saccharomyces cerevisiae: effects on cell growth and bud morphogenesis. Mol Cell Biol. 1991 Oct;11(10):4876–4884. doi: 10.1128/mcb.11.10.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampei G., Mizobuchi K. Nucleotide sequence of the Escherichia coli purF gene encoding amidophosphoribosyltransferase for de novo purine nucleotide synthesis. Nucleic Acids Res. 1988 Sep 12;16(17):8717–8717. doi: 10.1093/nar/16.17.8717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schekman R., Brawley V. Localized deposition of chitin on the yeast cell surface in response to mating pheromone. Proc Natl Acad Sci U S A. 1979 Feb;76(2):645–649. doi: 10.1073/pnas.76.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso J. Y., Zalkin H., van Cleemput M., Yanofsky C., Smith J. M. Nucleotide sequence of Escherichia coli purF and deduced amino acid sequence of glutamine phosphoribosylpyrophosphate amidotransferase. J Biol Chem. 1982 Apr 10;257(7):3525–3531. [PubMed] [Google Scholar]
- Walker J. E., Gay N. J., Saraste M., Eberle A. N. DNA sequence around the Escherichia coli unc operon. Completion of the sequence of a 17 kilobase segment containing asnA, oriC, unc, glmS and phoS. Biochem J. 1984 Dec 15;224(3):799–815. doi: 10.1042/bj2240799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watzele G., Tanner W. Cloning of the glutamine:fructose-6-phosphate amidotransferase gene from yeast. Pheromonal regulation of its transcription. J Biol Chem. 1989 May 25;264(15):8753–8758. [PubMed] [Google Scholar]



