Abstract
There is increased neuronal firing in the high vocal center (a motor nucleus) and other song nuclei of canaries, Serinus canaria, and zebra finches, Taeniopygia guttata, whenever these songbirds sing or hear song. These observations suggested that song perception involved sensory and motor pathways. We now show that the act of singing, but not hearing song, induces a rapid and striking increase (up to 60-fold) in expression of the transcriptional regulator ZENK in the high vocal center and other song nuclei. This motor-driven gene expression is independent of auditory feedback, since it occurs in deafened birds when they sing and in muted birds when they produce silent song. Conversely, hearing song, but not the act of singing, induces ZENK expression in parts of the auditory forebrain. Our observations show that even though the same auditory stimulus activates sensory and motor pathways, perception and production of song are accompanied by anatomically distinct patterns of gene expression.
Keywords: birdsong, vocalizations, perception, immediate early genes, ZENK
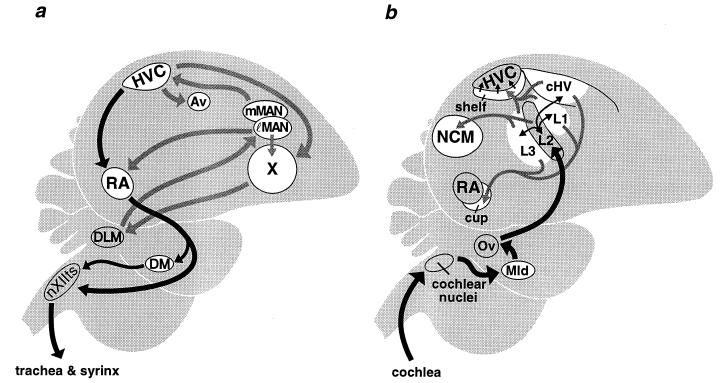
Canary ZENK is an acronym for the gene known in other species as Zif-268, Egr-1, NGFIA, and Krox-24 (1). ZENK and other immediate early gene transcription factors have been implicated in processes of synaptic plasticity involved in learning (2, 3). ZENK is induced in six auditory regions of caudal forebrain (ref. 4; Fig. 1b) when birds hear novel conspecific song (8, 9). However, though many attempts were made using different experimental paradigms, no expression was found in any of the song nuclei (Fig. 1a; refs. 4, 9, and 10) that are necessary for the acquisition and production of learned song. This observation puzzled us because playbacks of conspecific song induce depolarizing responses in song nuclei (11–15) and there are many instances in which membrane depolarization has been shown to induce ZENK expression (16). In addition, song nuclei show marked anatomical plasticity in adulthood (17–20) and therefore the absence of ZENK expression in them was doubly surprising. This study shows that the act of singing, induces ZENK expression in song nuclei.*
Figure 1.
Sagittal diagrams of songbird brain. (a) Anatomical relations between song nuclei (white) in which ZENK expression was induced by the act of singing. Black arrows show the direct motor pathway that innervates the vocal organs and produces learned song. Shaded arrows show links between other song system nuclei (5). The connections between RA and mMAN are described elsewhere (6). (b) Anatomical relations between forebrain auditory relays (white) in which ZENK expression was induced by hearing song. Thick black arrows show the major ascending auditory pathway, which ends in L2; thin black and gray arrows show some of the connections between forebrain auditory regions and the channeling of this input into HVC. Though not shown, NCM and cHV are reciprocally connected (7). Expression in the 12th and cochlear nuclei was not investigated. DLM, medial nucleus of dorsolateral thalamus; nXIIts, tracheosyringeal portion of the hypoglossal (12th) nucleus; Ov, nucleus ovoidalis. Other abbreviations are as used in the text.
MATERIALS AND METHODS
Animals.
Adult (1–2 years old) male canaries of the Belgian Waterslager strain and juvenile and adult male zebra finches from our breeding colonies were used. The number of birds used for each experiment is indicated in the text or figure legends.
Behavior. Thirty minute singing.
Groups of 4–6 male canaries were placed simultaneously in individual adjacent cages in a soundproof room for 24 hr to get them used to their new environment. The next morning, tape recorded song of another male canary, including the natural silent intervals between songs, was played for 30 min to stimulate them to sing. The number of songs produced by each male was counted from behind a one-way glass mirror. Whereas some birds engaged in counter-singing (hearing and singing) with the playback and among themselves, others did not sing during this time (hearing only). Birds were killed at various times during the 30-min singing period and after it ended.
Continuous singing.
For experiments that measured the persistence of ZENK expression in canaries singing for periods longer than 30 min, groups of 10–15 strong singers were taken from the aviary and placed into individual cages in a room. In addition to the playback, the greater number of birds involved stimulated the males to sing throughout the day. To encourage relatively constant singing and hearing levels for a full 6 hr, only a few birds (3–4) were removed and killed during this period. This experiment was repeated five times to get an n = 3 per sampling time. Only birds that sang a mean of 25–50 songs per hour were included in the sampling.
Baseline controls.
In addition to the hearing only group described above in the 30-min singing experiment, three other sets of intact canaries were used to evaluate ZENK expression in the absence of singing or hearing song: (i) birds in a quiet room that in the absence of playbacks naturally remained silent for at least 1 hr (silence), (ii) strong singers placed in a quiet room and prevented from singing for 1 hr by one of us sitting next to their cage, (iii) strong singers exposed to song and allowed to sing for 1 min after a 1-hr silent period.
Bird’s own song.
The song of individual canaries that sang 40 or more times in a 30-min period was recorded, and on the following day this recording was played back to the same bird while one of us sat next to its cage to discourage it from singing.
Deaf and mute singers.
Canaries were deafened by bilateral removal of the cochleas or muted by section of the left hypoglossal nerve, which is dominant in canary song production, using described protocols (21, 22). Birds in both groups were stimulated to sing by presenting them with other, intact, singing male canaries. Deafened canaries sang less and so were given testosterone implants (23) to induce them to sing more. In the absence of singing, testosterone treatment did not change basal levels of ZENK expression in auditory or song nuclei over that seen in untreated controls (not shown).
Seasonality.
Canaries were stimulated to sing, in the same manner as described for the 30-min group, for each month of the year (April 1995 to March 1996) before being killed.
Song development.
This experiment was done with zebra finches. Subsong and plastic song were elicited from juveniles by placing them near a window next to a cage that held an adult male and female 1 day prior to the experiment. Under these conditions, the juveniles would reliably sing the next morning at early dawn, often right after the adult male sang. Adult song was elicited in a similar manner as that described above for male canaries, except that in this case zebra finch song was used as a stimulus. All birds were killed 30 min after the onset of singing.
In Situ Hybridizations and Quantification.
Immediately after a bird was killed, its brain was removed and processed for in situ hybridization with a canary ZENK 35S-riboprobe using previously described protocols (9, 10). Gene expression was quantified without knowing the number of songs each bird had sung using W. S. Rasband’s 1996 National Institutes of Health–image computer analysis system. For each brain region the number of exposed silver grains in a 100 μ × 100 μ field in the middle of that structure was counted, the background subtracted, and the resulting value divided by the number of cells (neurons and glia); then the procedure was repeated on another section and from these two counts an average number of grains per cell was obtained. Depending on the region analyzed, this sampling included 70–120 cells. Normalization of the results for each experiment is described in the figure legends.
RESULTS
Singing vs. Hearing.
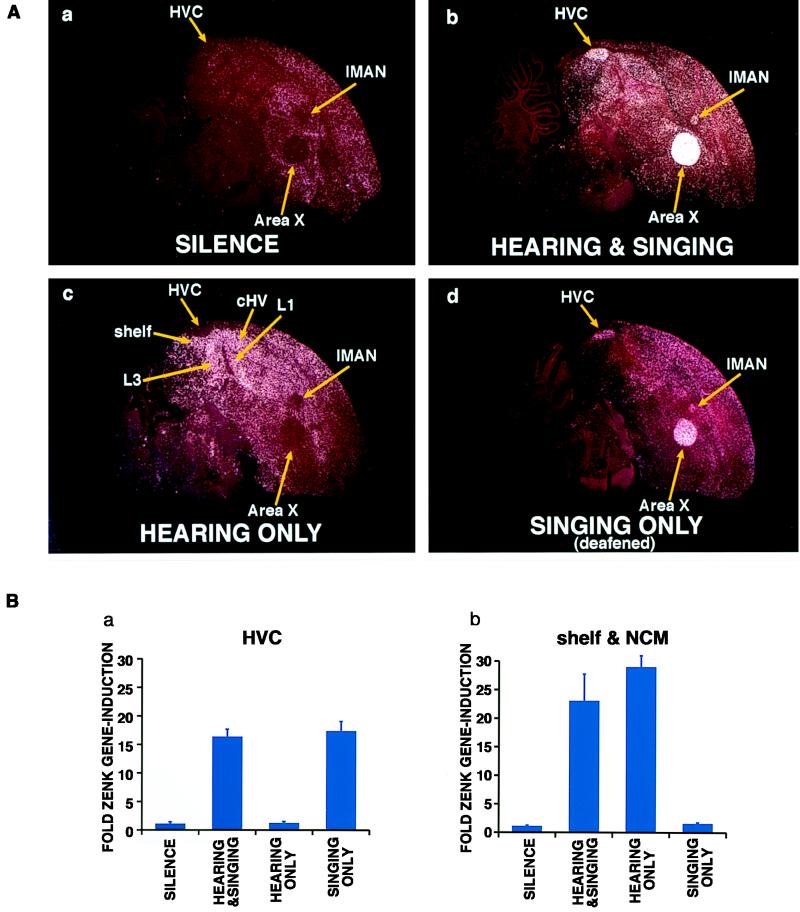
Figs. 2 and 3 show that canaries that were exposed to song and responded by singing had a 10- to 60-fold increase in ZENK gene expression in six of their forebrain song nuclei and in one midbrain song nucleus. These nuclei (refs. 25–27; Fig. 1a) were: (i) high vocal center (HVC), (ii) the robust nucleus of the archistriatum (RA), (iii) area X of lobus parolfactorius, (iv) the lateral magnocellular nucleus of the anterior neostriatum (lMAN), (v) its medial counterpart (mMAN), (vi) nucleus avalanche (Av) of the hyperstriatum ventrale, and (vii) the dorsomedial nucleus of the intercollicular complex (DM) (Fig. 2Ab shows ZENK expression in three of these nuclei). Birds that heard song and did or did not sing back had ZENK expression in seven auditory relays (Fig. 1b): the dorsolateral mesencephalic nucleus, L1, L3, the “shelf” under HVC, the “cup” apposed to RA, the caudal hyperstriatum ventrale (cHV), and the caudomedial neostriatum (NCM), as reported earlier for song playbacks (4). (Fig. 2A b and c shows ZENK expression in four of these regions.) Many of the cells that showed singing-induced ZENK expression were neurons because it was possible to backfill them, in HVC for example, with a retrograde tracer (rhodamine beads) injected into a downstream nucleus (E.D.J., C. Scharff, and F.N., unpublished data).
Figure 2.
ZENK expression in canary brain. (A) Darkfield view of cresyl-violet stained (red) brain sections taken 2 mm lateral from midline and reacted with a canary ZENK probe (white grains). At this medial-lateral level one can see a subset of structures (yellow arrows), as outlined in Fig. 1, that showed that singing or hearing song induced ZENK expression. (a) Bird exposed to silence and that did not sing, (b) bird that sang 61 songs during the 30-min song stimulation period, (c) bird in an adjacent cage that did not sing during the same 30-min period, (d) deafened bird that sang 30 songs during 30 min. (B) The effects seen in A are quantified here for a, one song nucleus (HVC) and b, combined but similar averages of two auditory relays (shelf and NCM), as representative examples. The y-axis shows ZENK expression relative to that in controls exposed to silence and that did not sing. For these comparisons, birds in the two groups in which singing occurred were matched for the number of songs (mean of 20 songs in 30 min). Mann–Whitney U test showed that all the obvious differences in these histograms were significant (P < 0.02; n = 4 per group, singing only, n = 3). Singing dampened the hearing song-induced ZENK expression in the shelf and NCM, but in this sample the difference was not significant (but see Fig. 3B). Error bars represent SEM.
Figure 3.
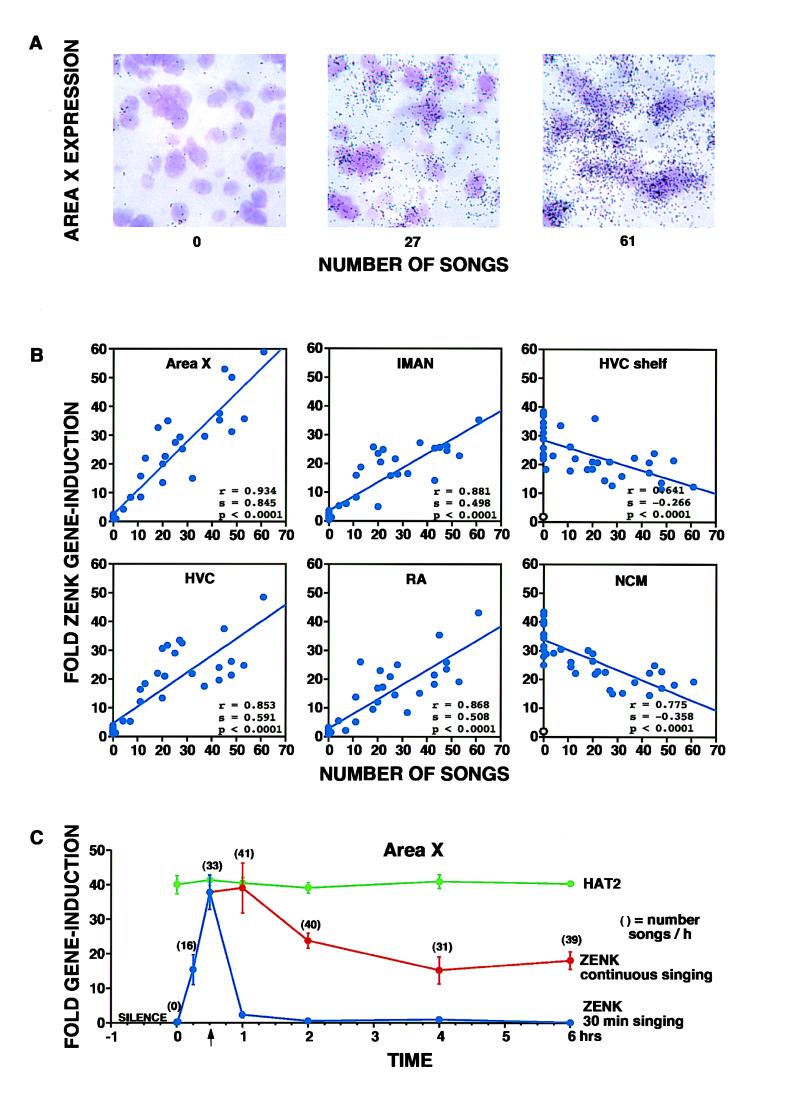
(A) ZENK expression in area X. Brightfield ×63 magnification shows that the amount of ZENK label (exposed silver grains, black) over cresyl-violet stained cells (purple) was proportional to the number of songs sung during a 30-min period. (B) Quantification of this correlation in four song nuclei (area X, lMAN, HVC, and RA) and in two auditory relays (HVC shelf and NCM), as representative examples. Each solid blue circle represents the value of one canary (n = 37; 22 birds that sang, 15 that did not). For song nuclei, each value was obtained by dividing the total number of grains per cell in a particular nucleus by the average number of grains per cell seen in that nucleus in the birds that did not sing (hearing only group). For auditory relays, the values were divided by the average of those seen in controls that were exposed to silence and did not sing (n = 3) (open circle at the bottom of the shelf and NCM graphs). The solid line through the dots represents the best linear fit of the data. Statistical values were determined by regression analysis. (C) Time course of gene expression in area X before, during, and after singing. ZENK values for strong singers prevented from singing for 1 hr or singing vigorously for 1 min thereafter are overlapped at time 0 with silent controls. Blue curve: ZENK expression in birds that sang for 15 min and then were killed, or for 30 min and then were interrupted (arrow) and killed immediately or at various times thereafter. Red curve: ZENK expression in birds that continued to sing and were killed at various times after the onset of singing. Levels of ZENK expression in this group remained higher than in those that were interrupted (P < 0.05 between 1–6 hr; two-tailed unpaired t test). Green curve: the expression of HAT2, a relatively abundant brain specific gene (24), did not change as a result of singing. Values in brackets represent the average number of songs per group per last hour of sampling except for the first two, which indicate the number of songs during the first 15 and 30 min, respectively (n = 3 birds per time period sampled). Error bars represent SEM.
Proportionality.
The amount of ZENK expression in song nuclei was linearly proportional to the number of songs sung per unit of time (Fig. 3A). In birds that did not sing at all expression was very low, those that sang intermediate amounts had intermediate levels of expression, and those that sang the most had the highest levels of expression. This correlation was strong (r = 0.853–0.934, P < 0.001; Fig. 3B). The slopes of the regression lines show that area X had the highest rate of singing-induced expression (s = 0.9-fold per song) and RA and lMAN the lowest (s = 0.5). ZENK expression induced in the “shelf” under HVC, in NCM, and in related auditory structures of all birds hearing song was down-regulated by singing (Figs. 2A, compare b with c, and 3B). Perhaps, as reported for middle ear muscles (28) and HVC neurons (11), singing dampened the response to sound.
Which Comes First:
ZENK or Singing? We wanted to know whether or not the ZENK expression seen in song nuclei preceded singing or was a consequence of singing. When strong singers were prevented from singing for 1 hr or allowed to sing vigorously for 1 min thereafter and then killed, ZENK expression in song nuclei was very low and no different from that in birds that were naturally silent (time 0 of Fig. 3C). However, if the birds were allowed to sing for 10–15 min, ZENK expression was readily detectable and peaked 30 min after the onset of singing. When singing was stopped at 30 min, by our presence again, expression rapidly declined, reaching nonsinging levels 1.5 hr later, and remained very low for at least the next 4 hr. When singing was allowed to continue past 30 min, instead of ZENK expression increasing further, it started to decline 1 hr after onset of singing and thereafter was maintained at a higher level than in birds that were not singing (Fig. 3C). Songs became shorter as singing progressed and this could account for some of the decreased expression seen under continuous singing. During this latter period, birds that sang more songs per hour still had higher levels than those that sang less. Expression of a constitutively expressed brain-specific gene, HAT2 (24), did not change during singing showing that the ZENK increase was specific and not a general gene expression response (Fig. 3C). These results suggest that singing induced ZENK expression in the brain of the singer, rather than the other way around. They also suggest that when singing persisted for several hours, ZENK mRNA was stabilized or continuously used up and then replaced.
Auditory Feedback.
It is well known that playbacks of a bird’s own song(s) are a more effective auditory stimulus than playbacks of other conspecific songs for driving the firing rate of song nuclei neurons (13–15). However, such playbacks did not induce ZENK expression in any of the song nuclei above the levels seen in birds exposed to silence (P ranged from 0.3 to 0.7 for different song nuclei, n = 4 per group; Mann–Whitney U test). Despite this observation, it was still possible that induction in song nuclei was dependent on the synchronous occurrence of the bird singing and hearing itself sing. To address this issue, we measured ZENK expression in deafened canaries. Although the deafened males sang less than most of the intact ones (an average of 20 songs per 30 min), ZENK expression in their song nuclei (Fig. 2Ad) was similar to that seen in intact males singing at comparable rates (Fig. 2Ba). In contrast, the ZENK expression normally induced in auditory regions by hearing song (Fig. 2Ac) was blocked in the deafened adult males whether or not they sang (Fig. 2 Ad and Bb). Thus, the act of singing did not, by itself, induce ZENK expression in the ascending auditory pathway. Therefore, all of the induced ZENK gene expression found in the song nuclei in the natural situation of hearing song and singing can be explained just by the act of singing. Conversely, all of the induced expression in the auditory relays can be explained just by hearing song.
Muting.
Even if auditory feedback was not necessary for singing-induced ZENK expression in song nuclei, proprioceptive feedback from the vocal organ could have played a role. To test for this possibility we muted birds by sectioning the left tracheosyringeal nerve. Birds operated in this manner and presented with another canary, “attempted” to sing, as inferred from their posture, quivering throat, and open bill, but only produced faint clicking sounds and silent vocal gestures. Yet the level of ZENK expression in their song nuclei was similar to that of intacts singing a comparable number of songs (P = 0.2, n = 3 and 4, respectively; Mann–Whitney U test, same P for HVC and area X). Thus, the motor act of singing in the absence of normal sound production—and even in the presence of altered proprioceptive feedback—is sufficient for singing-induced ZENK expression in song nuclei.
Vocal Learning.
Because of its suggested role in learning and memory (3, 8, 9), we wondered whether the level of singing-induced ZENK expression in song nuclei might be correlated with natural periods of song learning. Male canaries continue to modify their song in adulthood, with most changes occurring in late summer and early fall (29). Despite this seasonality in song learning, the anatomical distribution and level of ZENK expression induced by singing remained comparable throughout the year; for example, the 0.9-fold increase in area X per song sung was remarkably stable from month to month (n = 3–6 per month, P = 0.82, ANOVA).
Another songbird, the zebra finch, learns its song only once during a sensitive period preceding sexual maturity (30). In zebra finches the basal level of ZENK in the forebrain (including song nuclei) is higher in juveniles than in adults (31). Yet the relative increase in ZENK expression in area X and HVC—above that seen in nonsinging controls of the same age—was comparable in juvenile male zebra finches singing subsong (age = 35 and 36 days; n = 2 singing, 2 controls) or plastic song (age = 50–65 days; n = 5 singing, 3 controls), and in adult zebra finches singing stable adult song (age = 90 days to 4.5 years; n = 16 singing, 6 controls; P = 0.36, ANOVA). Overall, the anatomical distribution and singing-induced changes in ZENK expression in the song nuclei of juvenile and adult zebra finches were similar to those seen in adult canaries.
DISCUSSION
We believe this to be the most striking example of motor driven gene expression generated by a natural, spontaneous behavior. An earlier report showed a 2-fold increase in c-fos expression in the motor cortex of rats performing a well-rehearsed, complex motor task. This increase was higher (3-fold) during the acquisition phase of this motor skill (32). By contrast, singing induced up to a 60-fold increase in ZENK expression, and this level was not affected by the extent to which the song produced had been mastered.
The singing-induced expression in area X and lMAN of adult male zebra finches is paradoxical because both nuclei are necessary for song learning in juveniles but can be removed in adult males without noticeable effects on the production of learned song (33–35). Furthermore, no electrophysiological activity changes have been detected in these nuclei during singing (36). By contrast, our observations suggest that area X and lMAN are very active during song production, though apparently in ways and with consequences that remain to be recognized. For students of birdsong, this is a most intriguing observation.
Fig. 1 summarizes the anatomical findings of this study. It shows a clear separation of expression into two set of areas, one where ZENK expression is triggered by song as a motor act and another in which expression is triggered by song as an auditory stimulus. This separation is remarkable because songs heard induce electrophysiological responses in both sets of areas (11–15, 37–40). We suggest that when auditory and song circuits respond electrophysiologically to songs heard, both circuits process the signals, as has been proposed in the motor theory of song perception (12), but the auditory circuit stores this information in a more lasting manner through changes in gene expression (1, 38). Likewise, when the song circuit fires during singing (11, 36, 41), it then is affected through gene expression in a more lasting manner, whereas in this case the auditory circuit remains unaffected. Perhaps it is important to segregate the genomic consequences of sensory and motor experience, particularly if each of these experiences can lead to memory formation. Otherwise, songs heard could alter directly the pathways used to produce song.
Results from our gene expression study complement observations made in humans. Experiments using positron-emission tomography showed that there is increased activity in both Broca’s (motor) and Wernicke’s (auditory) areas when people hear speech. Broca’s area also shows increased activity during speech production (42). Thus, at the level of changes in neurophysiological activity, there are parallels between human and songbird perception and production of vocal signals. However, the noninvasive methods used to image brain activity in humans do not tell us what might be the effects at the genomic level, an issue that we are now able to address with an animal model.
Observations in other systems (2, 3, 16, 32), as well as in songbird NCM (8, 9, 38), are compatible with the hypothesis that immediate early genes play a role in long-term memory formation, but the nature of this causal link remains hypothetical. Thus, we are left with the question of, what is the role of ZENK in the song nuclei, which are involved in the production of a stereotyped, well-learned behavior? We suggest two hypothesis: (i) that ZENK is involved in the replacement of proteins that get “used up” during singing, which implies that the half-life of these proteins is short and (ii) that ZENK is involved in the synthesis of proteins that strengthen the motor memory of a song every time it is sung, a process of “production-dependent learning” similar to Marler and Nelson’s “action-based learning” (43); these hypothesis are not mutually exclusive. Much as mature athletes and musicians exercise constantly to maintain the precision of their skill, so too, an adult songbird may learn a little every time it sings, perhaps to maintain what it knows.
Acknowledgments
We thank Emy Concepcion (Union Hill High School, New Jersey) for assistance in the beginning stages of this project. We thank Claudio Mello, Marta Nottebohm, Miriam Rivas, and Constance Scharff for useful discussions and critical comments on this manuscript. C. Mello alerted us to ZENK expression in DM (Fig. 1a). This research was supported by National Institute of Mental Health Postdoctoral Research Training and Rockefeller Minority Research Grants to E.D.J., by a National Institute of Mental Health Grant to F.N., and by the kind generosity of Mr. Herbert Singer, Mr. Howard Phipps, and the Mary Flagler Cary Charitable Trust.
ABBREVIATIONS
- HVC
high vocal center
- NCM
caudomedial neostriatum
- RA
archistriatum
- lMAN
lateral magnocellular nucleus of the anterior neostriatum
Note
While this work was being prepared for publication, we learned that another group (44) had found similar, but not identical, large changes in the expression of another immediate early gene, c-fos, in the song system of singing zebra fiches.
Footnotes
This work was presented in part at the 25th Annual Meeting for the Society of Neuroscience, San Diego, CA, November 11–16, 1995, and again at the Learning and Memory Meeting, Cold Spring Harbor, NY, October 2–6, 1996.
References
- 1.Mello C V, Vicario D S, Clayton D F. Proc Natl Acad Sci USA. 1992;89:6818–6822. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rose S P R. Trends Neurosci. 1991;14:390–397. doi: 10.1016/0166-2236(91)90027-r. [DOI] [PubMed] [Google Scholar]
- 3.Kaczmarek L. J Neurosci Res. 1993;34:377–381. doi: 10.1002/jnr.490340402. [DOI] [PubMed] [Google Scholar]
- 4.Mello C V, Clayton D F. J Neurosci. 1994;14:6652–6666. doi: 10.1523/JNEUROSCI.14-11-06652.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vates G E, Nottebohm F. Proc Natl Acad Sci USA. 1995;92:5139–5143. doi: 10.1073/pnas.92.11.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vates G E, Vicario D S, Nottebohm F. J Comp Neurol. 1997;380:275–290. [PubMed] [Google Scholar]
- 7.Vates G E, Broome B M, Mello C V, Nottebohm F. J Comp Neurol. 1996;366:613–642. doi: 10.1002/(SICI)1096-9861(19960318)366:4<613::AID-CNE5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 8.Mello C V, Nottebohm F, Clayton D F. J Neurosci. 1995;15:6919–6925. doi: 10.1523/JNEUROSCI.15-10-06919.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jarvis E D, Mello C V, Nottebohm F. Learn Mem. 1995;2:62–80. doi: 10.1101/lm.2.2.62. [DOI] [PubMed] [Google Scholar]
- 10.Mello C V, Clayton D F. J Neurobiol. 1995;26:145–161. doi: 10.1002/neu.480260112. [DOI] [PubMed] [Google Scholar]
- 11.McCasland J S, Konishi M. Proc Natl Acad Sci USA. 1981;78:7815–7819. doi: 10.1073/pnas.78.12.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams H, Nottebohm F. Science. 1985;229:279–282. doi: 10.1126/science.4012321. [DOI] [PubMed] [Google Scholar]
- 13.Margoliash D. J Neurosci. 1986;6:1643–1661. doi: 10.1523/JNEUROSCI.06-06-01643.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doupe A J, Konishi M. Proc Natl Acad Sci USA. 1991;88:11339–11343. doi: 10.1073/pnas.88.24.11339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vicario D S, Yohay K H. J Neurobiol. 1993;24:488–505. doi: 10.1002/neu.480240407. [DOI] [PubMed] [Google Scholar]
- 16.Hughs O, Drugunow M. Pharmacol Rev. 1995;37:133–177. [Google Scholar]
- 17.DeVoogd T J, Nottebohm F. Science. 1981;214:202–204. doi: 10.1126/science.7280692. [DOI] [PubMed] [Google Scholar]
- 18.Nottebohm F. Science. 1981;214:1368–1370. doi: 10.1126/science.7313697. [DOI] [PubMed] [Google Scholar]
- 19.Nordeen K W, Nordeen E J. Nature (London) 1988;334:149–151. doi: 10.1038/334149a0. [DOI] [PubMed] [Google Scholar]
- 20.Alvarez-Buylla A, Theelen M, Nottebohm F. Proc Natl Acad Sci USA. 1988;85:8722–8726. doi: 10.1073/pnas.85.22.8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konishi M. Z Tierpsychol. 1965;22:770–783. [PubMed] [Google Scholar]
- 22.Nottebohm F, Nottebohm M E. J Comp Physiol A. 1976;108:171–192. [Google Scholar]
- 23.Nottebohm F. Brain Res. 1980;192:89–107. doi: 10.1016/0006-8993(80)91011-2. [DOI] [PubMed] [Google Scholar]
- 24.George J, Clayton D F. Mol Brain Res. 1992;12:323–329. doi: 10.1016/0169-328x(92)90134-w. [DOI] [PubMed] [Google Scholar]
- 25.Nottebohm F, Stokes T M, Leonard C M. J Comp Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- 26.Nottebohm F, Kelley D B, Paton J A. J Comp Neurol. 1982;207:344–357. doi: 10.1002/cne.902070406. [DOI] [PubMed] [Google Scholar]
- 27.Bottjer S W, Halsema K A, Brown S A, Miesner E A. J Comp Neurol. 1989;279:312–326. doi: 10.1002/cne.902790211. [DOI] [PubMed] [Google Scholar]
- 28.Borg E, Counter S A. Sci Am. 1989;261:74–80. doi: 10.1038/scientificamerican0889-74. [DOI] [PubMed] [Google Scholar]
- 29.Nottebohm F, Nottebohm M E, Crane L. Behav Neural Biol. 1986;46:445–471. doi: 10.1016/s0163-1047(86)90485-1. [DOI] [PubMed] [Google Scholar]
- 30.Immelmann K. In: Bird Vocalizations. Hinde R A, editor. London: Cambridge Univ. Press; 1969. pp. 61–74. [Google Scholar]
- 31.Mello C V. Ph.D. thesis. New York: The Rockefeller University; 1993. [Google Scholar]
- 32.Kliem J A, Lussnig E, Schwarz E R, Comery T A, Greenough W T. J Neurosci. 1996;16:4529–4535. doi: 10.1523/JNEUROSCI.16-14-04529.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bottjer S W, Miesner E A, Arnold A P. Science. 1984;224:901–903. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- 34.Sohrabji F, Nordeen E J, Nordeen K W. Behav Neural Biol. 1990;53:51–63. doi: 10.1016/0163-1047(90)90797-a. [DOI] [PubMed] [Google Scholar]
- 35.Scharff C, Nottebohm F. J Neurosci. 1991;11:2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCasland J S. J Neurosci. 1987;7:23–29. doi: 10.1523/JNEUROSCI.07-01-00023.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams H. Ann NY Acad Sci. 1989;563:148–164. [Google Scholar]
- 38.Chew S J, Vicario D S, Nottebohm F. Science. 1996;274:1909–1914. doi: 10.1126/science.274.5294.1909. [DOI] [PubMed] [Google Scholar]
- 39.Leppelsack H-J. In: Advances in Vertebrate Neuroethology. Ewert J-P, Capranica R R, Ingle D J, editors. New York: Plenum; 1983. pp. 783–799. [Google Scholar]
- 40.Katz L C, Gurney M E. Brain Res. 1981;221:192–197. doi: 10.1016/0006-8993(81)91073-8. [DOI] [PubMed] [Google Scholar]
- 41.Yu A C, Margoliash D. Science. 1996;273:1871–1875. doi: 10.1126/science.273.5283.1871. [DOI] [PubMed] [Google Scholar]
- 42.Price C J, Wise R J S, Warburton E A, Moore C J, Howard D, Patterson K, Frackowiak R S J, Friston K J. Brain. 1996;119:919–931. doi: 10.1093/brain/119.3.919. [DOI] [PubMed] [Google Scholar]
- 43.Marler P, Nelson D A. Proc Natl Acad Sci USA. 1994;91:10498–10501. doi: 10.1073/pnas.91.22.10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kimbo R, Doupe A. Soc Neurosci Abstr. 1996;22:691. [Google Scholar]