Abstract
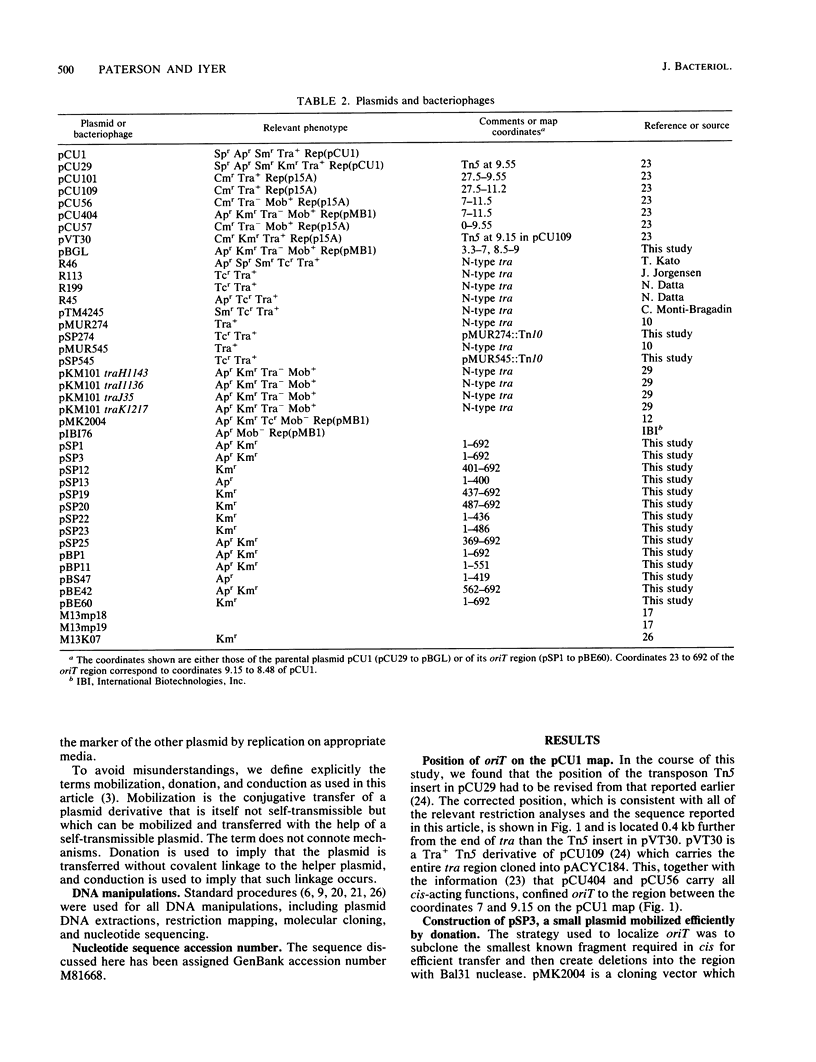
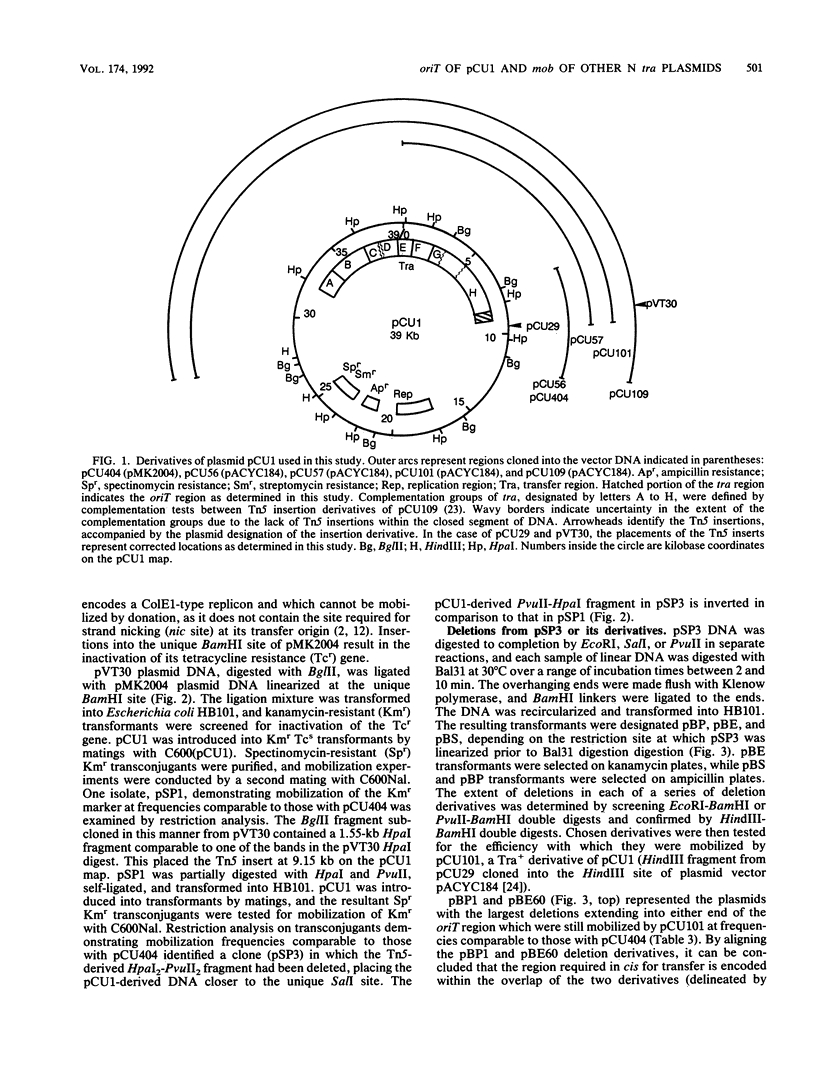
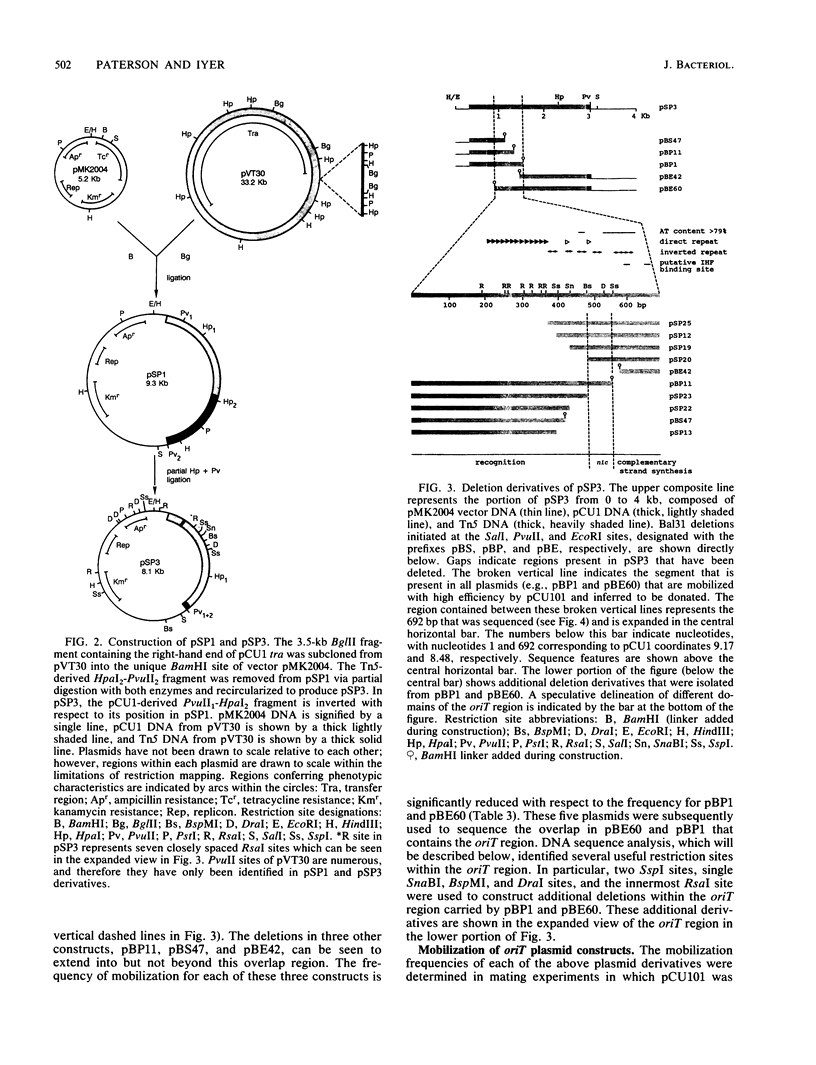
The oriT region of the conjugative IncN plasmid pCU1 has been localized to a 669-bp sequence extending from pCU1 coordinates 8.48 to 9.15 kb. The nucleotide sequence of this region was determined. The region is AT-rich (69% AT residues), with one 19-bp and one 81-bp sequence containing 79% or more AT residues. Prominent sequence features include one set of thirteen 11-bp direct repeats, a second set of two 14-bp direct repeats, six different inverted repeat sequences ranging from 6 to 10 bp in size, and two sequences showing 12 of 13 nucleotides identical to the consensus integration host factor binding sequence. Specificity between this oriT and mobilization (mob) functions encoded by the N tra system was demonstrated. This specificity is encoded by the region lying clockwise of the BglII site at coordinate 3.3 on the pCU1 map. Two N tra plasmids isolated in the preantibiotic era were unable to mobilize recombinant plasmids carrying the oriT region of pCU1 or to complement transposon Tn5 mutations in the mob region of the closely related plasmid pKM101.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastia D. Determination of restriction sites and the nucleotide sequence surrounding the relaxation site of ColE1. J Mol Biol. 1978 Oct 5;124(4):601–639. doi: 10.1016/0022-2836(78)90174-2. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Clark A. J., Warren G. J. Conjugal transmission of plasmids. Annu Rev Genet. 1979;13:99–125. doi: 10.1146/annurev.ge.13.120179.000531. [DOI] [PubMed] [Google Scholar]
- Coupland G. M., Brown A. M., Willetts N. S. The origin of transfer (oriT) of the conjugative plasmid R46: characterization by deletion analysis and DNA sequencing. Mol Gen Genet. 1987 Jun;208(1-2):219–225. doi: 10.1007/BF00330445. [DOI] [PubMed] [Google Scholar]
- Dempsey W. B. Integration host factor and conjugative transfer of the antibiotic resistance plasmid R100. J Bacteriol. 1987 Sep;169(9):4391–4392. doi: 10.1128/jb.169.9.4391-4392.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt T. A rapid method for the identification of plasmid desoxyribonucleic acid in bacteria. Plasmid. 1978 Sep;1(4):584–588. doi: 10.1016/0147-619x(78)90016-1. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Frost L. S., Paranchych W. Origin of transfer of IncF plasmids and nucleotide sequences of the type II oriT, traM, and traY alleles from ColB4-K98 and the type IV traY allele from R100-1. J Bacteriol. 1986 Oct;168(1):132–139. doi: 10.1128/jb.168.1.132-139.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürste J. P., Pansegrau W., Ziegelin G., Kröger M., Lanka E. Conjugative transfer of promiscuous IncP plasmids: interaction of plasmid-encoded products with the transfer origin. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1771–1775. doi: 10.1073/pnas.86.6.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hughes V. M., Datta N. Conjugative plasmids in bacteria of the 'pre-antibiotic' era. Nature. 1983 Apr 21;302(5910):725–726. doi: 10.1038/302725a0. [DOI] [PubMed] [Google Scholar]
- Kahn M., Kolter R., Thomas C., Figurski D., Meyer R., Remaut E., Helinski D. R. Plasmid cloning vehicles derived from plasmids ColE1, F, R6K, and RK2. Methods Enzymol. 1979;68:268–280. doi: 10.1016/0076-6879(79)68019-9. [DOI] [PubMed] [Google Scholar]
- Konarska-Kozlowska M., Iyer V. N. The genetic and physical basis of variability in Escherichia coli strains carrying a reference Inc N group plasmid. Can J Microbiol. 1981 Jun;27(6):616–626. doi: 10.1139/m81-094. [DOI] [PubMed] [Google Scholar]
- Leong J. M., Nunes-Düby S., Lesser C. F., Youderian P., Susskind M. M., Landy A. The phi 80 and P22 attachment sites. Primary structure and interaction with Escherichia coli integration host factor. J Biol Chem. 1985 Apr 10;260(7):4468–4477. [PubMed] [Google Scholar]
- Lovett M. A., Guiney D. G., Helinski D. R. Relaxation complexes of plasmids ColE1 and ColE2: unique site of the nick in the open circular DNA of the relaxed complexes. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3854–3857. doi: 10.1073/pnas.71.10.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Hashimoto-Gotoh T., Timmis K. N. Location of two relaxation nick sites in R6K and single sites in pSC101 and RSF1010 close to origins of vegetative replication: implication for conjugal transfer of plasmid deoxyribonucleic acid. J Bacteriol. 1980 Dec;144(3):923–932. doi: 10.1128/jb.144.3.923-932.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer H. E., Sederoff R. R. Improved estimation of DNA fragment lengths from Agarose gels. Anal Biochem. 1981 Jul 15;115(1):113–122. doi: 10.1016/0003-2697(81)90533-9. [DOI] [PubMed] [Google Scholar]
- Snijders A., van Putten A. J., Veltkamp E., Nijkamp H. J. Localization and nucleotide sequence of the bom region of Clo DF13. Mol Gen Genet. 1983;192(3):444–451. doi: 10.1007/BF00392189. [DOI] [PubMed] [Google Scholar]
- Thatte V., Bradley D. E., Iyer V. N. N conjugative transfer system of plasmid pCU1. J Bacteriol. 1985 Sep;163(3):1229–1236. doi: 10.1128/jb.163.3.1229-1236.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatte V., Iyer V. N. Cloning of a plasmid region specifying the N transfer system of bacterial conjugation in Escherichia coli. Gene. 1983 Mar;21(3):227–236. doi: 10.1016/0378-1119(83)90006-9. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Willetts N., Maule J. Specificities of IncF plasmid conjugation genes. Genet Res. 1986 Feb;47(1):1–11. doi: 10.1017/s0016672300024447. [DOI] [PubMed] [Google Scholar]
- Willetts N., Wilkins B. Processing of plasmid DNA during bacterial conjugation. Microbiol Rev. 1984 Mar;48(1):24–41. doi: 10.1128/mr.48.1.24-41.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans S. C., Walker G. C. Conjugal transfer system of the IncN plasmid pKM101. J Bacteriol. 1985 Jan;161(1):402–410. doi: 10.1128/jb.161.1.402-410.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakobson E., Guiney G. Homology in the transfer origins of broad host range IncP plasmids: definition of two subgroups of P plasmids. Mol Gen Genet. 1983;192(3):436–438. doi: 10.1007/BF00392187. [DOI] [PubMed] [Google Scholar]