Abstract
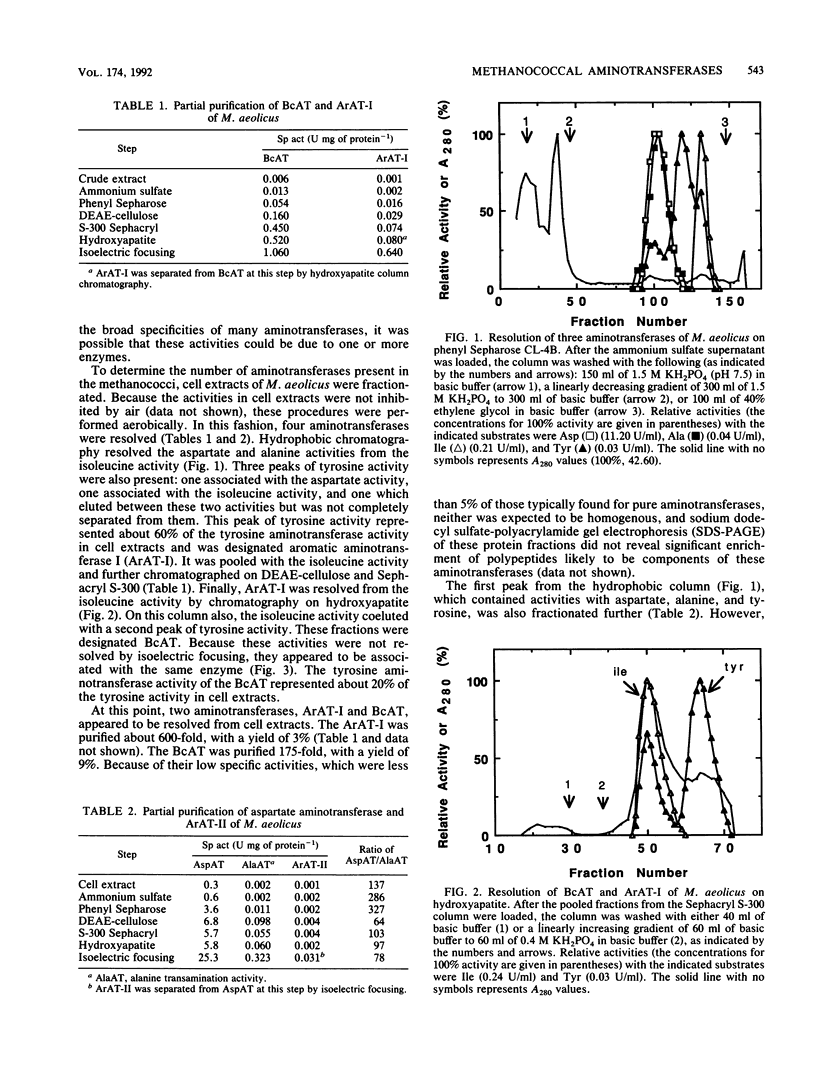
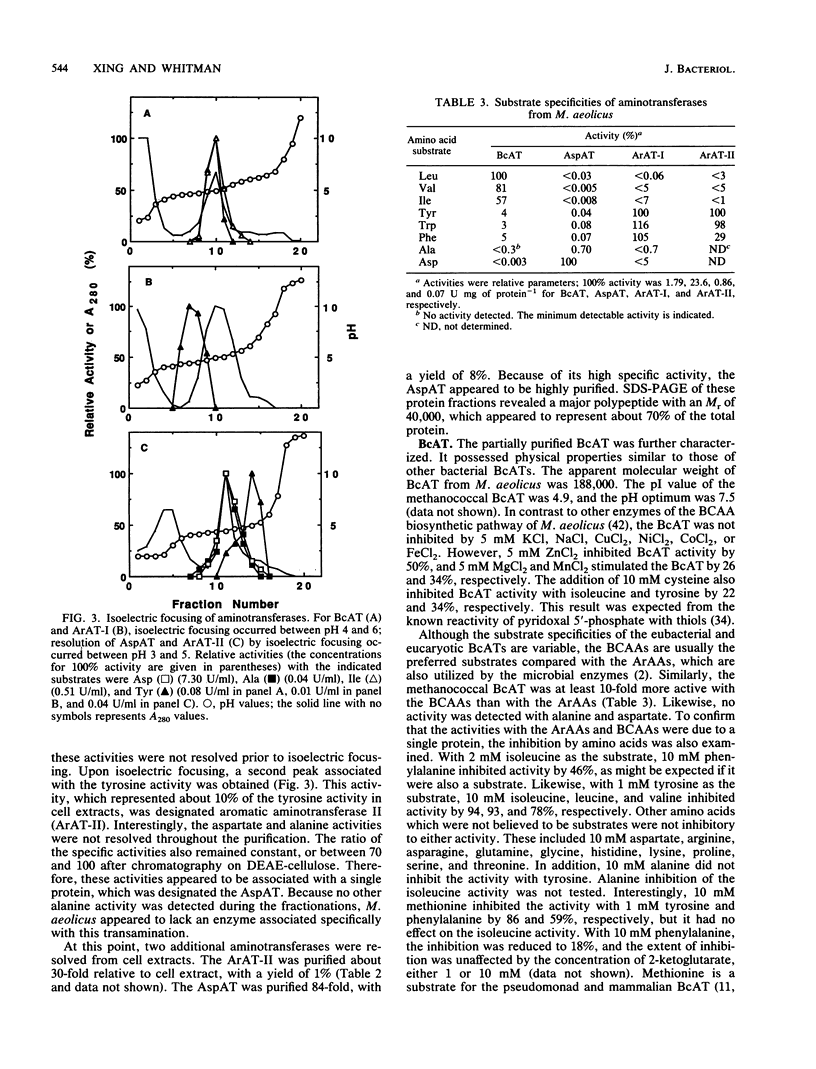
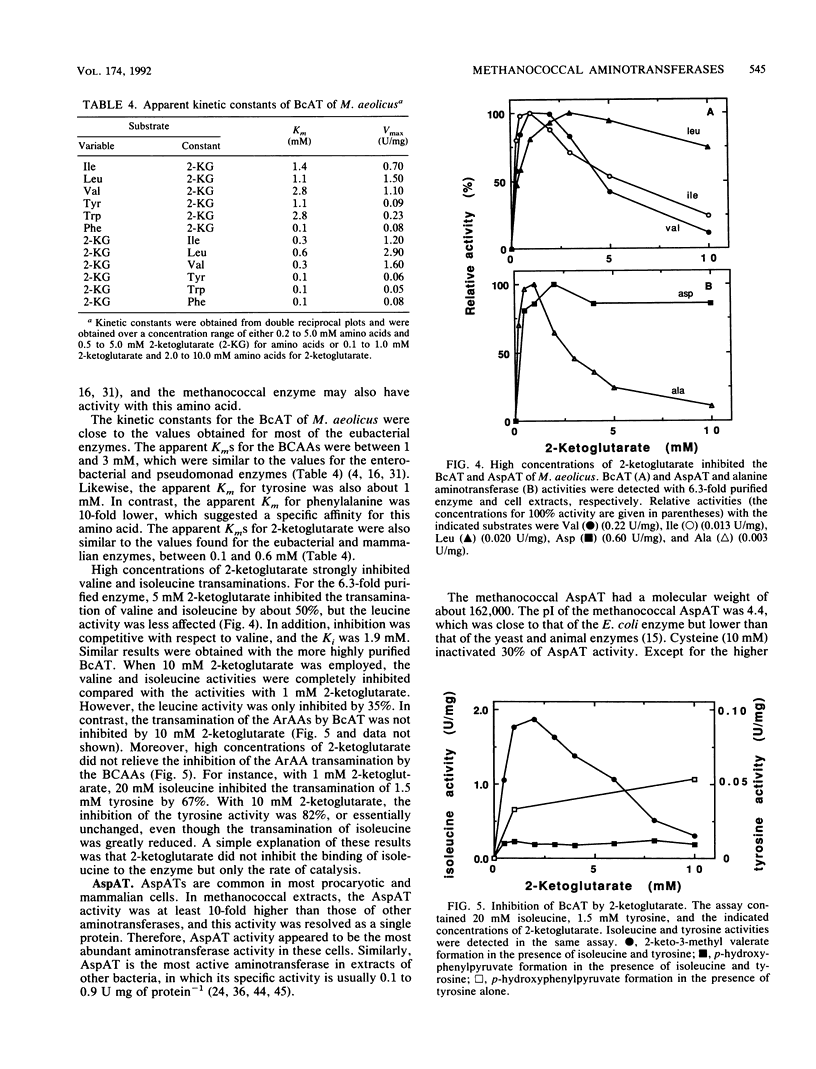
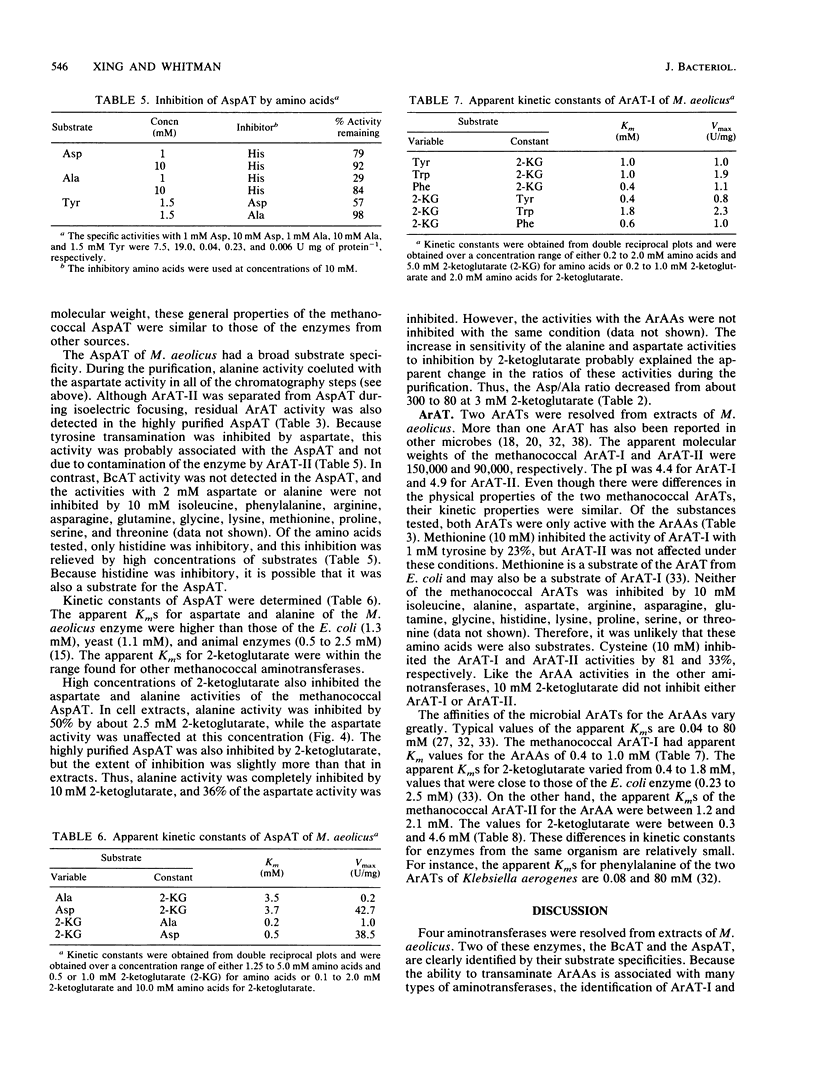
Four aminotransferases were identified and characterized from Methanococcus aeolicus. Branched-chain aminotransferase (BcAT, EC 2.6.1.42), aspartate aminotransferase (AspAT, EC 2.6.1.1), and two aromatic aminotransferases (EC 2.6.1.57) were partially purified 175-, 84-, 600-, and 30-fold, respectively. The apparent molecular weight, substrate specificity, and kinetic properties of the BcAT were similar to those of other microbial BcATs. The AspAT had an apparent molecular weight of 162,000, which was unusually high. It had also a broad substrate specificity, which included activity towards alanine, a property which resembled the enzyme from Sulfolobus solfataricus. An additional alanine aminotransferase was not found in M. aeolicus, and this activity of AspAT could be physiologically significant. The apparent molecular weights of the aromatic aminotransferases (ArAT-I and ArAT-II) were 150,000 and 90,000, respectively. The methanococcal ArATs also had different pIs and kinetic constants. ArAT-I may be the major ArAT in methanococci. High concentrations of 2-ketoglutarate strongly inhibited valine, isoleucine, and alanine transaminations but were less inhibitory for leucine and aspartate transaminations. Aromatic amino acid transaminations were not inhibited by 2-ketoglutarate. 2-Ketoglutarate may play an important role in the regulation of amino acid biosynthesis in methanococci.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chesne S., Pelmont J. Glutamate-oxaloacétate transaminase d'Escherichia coli. I. Purification et spécificité. Biochimie. 1973;55(3):237–244. doi: 10.1016/s0300-9084(73)80121-x. [DOI] [PubMed] [Google Scholar]
- Coleman M. S., Soucie W. G., Armstrong F. B. Branched chain amino acid aminotransferase of Salmonella typhimurium. II. Kinetic comparison with the enzyme from Salmonella montevideo. J Biol Chem. 1971 Mar 10;246(5):1310–1312. [PubMed] [Google Scholar]
- Collier R. H., Kohlhaw G. Nonidentity of the aspartate and the aromatic aminotransferase components of transaminase A in Escherichia coli. J Bacteriol. 1972 Oct;112(1):365–371. doi: 10.1128/jb.112.1.365-371.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubellis M. V., Rozzo C., Nitti G., Arnone M. I., Marino G., Sannia G. Cloning and sequencing of the gene coding for aspartate aminotransferase from the thermoacidophilic archaebacterium Sulfolobus solfataricus. Eur J Biochem. 1989 Dec 8;186(1-2):375–381. doi: 10.1111/j.1432-1033.1989.tb15219.x. [DOI] [PubMed] [Google Scholar]
- Ekiel I., Jarrell K. F., Sprott G. D. Amino acid biosynthesis and sodium-dependent transport in Methanococcus voltae, as revealed by 13C NMR. Eur J Biochem. 1985 Jun 3;149(2):437–444. doi: 10.1111/j.1432-1033.1985.tb08944.x. [DOI] [PubMed] [Google Scholar]
- Gelfand D. H., Steinberg R. A. Escherichia coli mutants deficient in the aspartate and aromatic amino acid aminotransferases. J Bacteriol. 1977 Apr;130(1):429–440. doi: 10.1128/jb.130.1.429-440.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENSON C. P., CLELAND W. W. KINETIC STUDIES OF GLUTAMIC OXALOACETIC TRANSAMINASE ISOZYMES. Biochemistry. 1964 Mar;3:338–345. doi: 10.1021/bi00891a007. [DOI] [PubMed] [Google Scholar]
- JACOBY G. A., LADU B. N. STUDIES ON THE SPECIFICITY OF TYROSINE-ALPHA-KETOGLUTARATE TRANSAMINASE. J Biol Chem. 1964 Feb;239:419–424. [PubMed] [Google Scholar]
- Jack G. W., McMahon P. C. A bacterial phenylalanine aminotransferase lacking pyridoxal 5'-phosphate as cofactor. Biochim Biophys Acta. 1978 Apr 12;523(2):344–357. doi: 10.1016/0005-2744(78)90037-2. [DOI] [PubMed] [Google Scholar]
- Kagamiyama H., Kondo K., Yagi T. Aspartate aminotransferase from E. coli--a comparative study among E. coli, and yeast enzymes and pig heart isozymes. Prog Clin Biol Res. 1984;144B:293–302. [PubMed] [Google Scholar]
- Kondo K., Wakabayashi S., Kagamiyama H. Structural studies on aspartate aminotransferase from Escherichia coli. Covalent structure. J Biol Chem. 1987 Jun 25;262(18):8648–8657. [PubMed] [Google Scholar]
- Kradolfer P., Niederberger P., Hütter R. Tryptophan degradation in Saccharomyces cerevisiae: characterization of two aromatic aminotransferases. Arch Microbiol. 1982 Dec 11;133(3):242–248. doi: 10.1007/BF00415010. [DOI] [PubMed] [Google Scholar]
- Lain-Guelbenzu B., Muñoz-Blanco J., Cárdenas J. Purification and properties of L-aspartate aminotransferase of Chlamydomonas reinhardtii. Eur J Biochem. 1990 Mar 30;188(3):529–533. doi: 10.1111/j.1432-1033.1990.tb15432.x. [DOI] [PubMed] [Google Scholar]
- Lee-Peng F. C., Hermodson M. A., Kohlhaw G. B. Transaminase B from Escherichia coli: quaternary structure, amino-terminal sequence, substrate specificity, and absence of a separate valine-alpha-ketoglutarate activity. J Bacteriol. 1979 Aug;139(2):339–345. doi: 10.1128/jb.139.2.339-345.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscomb E. L., Horton H. R., Armstrong F. B. Molecular weight, subunit structure, and amino acid composition of the branched chain amino acid aminotransferase of Salmonella typhimurium. Biochemistry. 1974 May 7;13(10):2070–2077. doi: 10.1021/bi00707a011. [DOI] [PubMed] [Google Scholar]
- Lowe P. N., Rowe A. F. Aspartate: 2-oxoglutarate aminotransferase from trichomonas vaginalis. Identity of aspartate aminotransferase and aromatic amino acid aminotransferase. Biochem J. 1985 Dec 15;232(3):689–695. doi: 10.1042/bj2320689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino G., Nitti G., Arnone M. I., Sannia G., Gambacorta A., De Rosa M. Purification and characterization of aspartate aminotransferase from the thermoacidophilic archaebacterium Sulfolobus solfataricus. J Biol Chem. 1988 Sep 5;263(25):12305–12309. [PubMed] [Google Scholar]
- Mavrides C., Comerton M. Aminotransferases for aromatic amino acids and aspartate in Bacillus subtilis. Biochim Biophys Acta. 1978 May 11;524(1):60–67. doi: 10.1016/0005-2744(78)90103-1. [DOI] [PubMed] [Google Scholar]
- Mavrides C., Orr W. Multispecific aspartate and aromatic amino acid aminotransferases in Escherichia coli. J Biol Chem. 1975 Jun 10;250(11):4128–4133. [PubMed] [Google Scholar]
- Mavrides C. Transamination of aromatic amino acids in Escherichia coli. Methods Enzymol. 1987;142:253–267. doi: 10.1016/s0076-6879(87)42035-1. [DOI] [PubMed] [Google Scholar]
- McGilvray D., Umbarger H. E. Regulation of transaminase C synthesis in Escherichia coli: conditional leucine auxotrophy. J Bacteriol. 1974 Nov;120(2):715–723. doi: 10.1128/jb.120.2.715-723.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier N., Montmitonnet A., Chesne S., Pelmont J. Transaminase B d'Escherichia coli. I. - Purification et premières propriétés. Biochimie. 1976;58(6):663–675. doi: 10.1016/s0300-9084(76)80390-2. [DOI] [PubMed] [Google Scholar]
- Noguchi T., Takada Y. The appearance of a new aromatic aminotransferase in the small intestines of vitamin B6-deficient rats. J Biol Chem. 1980 Oct 25;255(20):9844–9847. [PubMed] [Google Scholar]
- Norton J. E., Sokatch J. R. Purification and partial characterization of the branched chain amino acid transaminase of Pseudomonas aeruginosa. Biochim Biophys Acta. 1970 May 13;206(2):261–269. doi: 10.1016/0005-2744(70)90109-9. [DOI] [PubMed] [Google Scholar]
- Paris C. G., Magasanik B. Purification and properties of aromatic amino acid aminotransferase from Klebsiella aerogenes. J Bacteriol. 1981 Jan;145(1):266–271. doi: 10.1128/jb.145.1.266-271.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J. T., Morrison J. F. The purification and properties of the aspartate aminotransferase and aromatic-amino-acid aminotransferase from Escherichia coli. Eur J Biochem. 1978 Jun 15;87(2):391–400. doi: 10.1111/j.1432-1033.1978.tb12388.x. [DOI] [PubMed] [Google Scholar]
- Schonbeck N. D., Skalski M., Shafer J. A. Reactions of pyrzdoxal 5'-phosphate, 6-aminocaproic acid, cysteine, and penicilamine. Models for reactions of Schiff base linkages in pyridoxal 5'-phosphate-requiring enymes. J Biol Chem. 1975 Jul 25;250(14):5343–5351. [PubMed] [Google Scholar]
- Siegel W. H., Donohue T., Bernlohr R. W. Determination of pools of tricarboxylic acid cycle and related acids in bacteria. Appl Environ Microbiol. 1977 Nov;34(5):512–517. doi: 10.1128/aem.34.5.512-517.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen W. A., Berg C. M. Analysis of an avtA::Mu d1(Ap lac) mutant: metabolic role of transaminase C. J Bacteriol. 1982 May;150(2):739–746. doi: 10.1128/jb.150.2.739-746.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker R. J., Gaines C. G., Jensen R. A. A multispecific quintet of aromatic aminotransferases that overlap different biochemical pathways in Pseudomonas aeruginosa. J Biol Chem. 1982 Nov 25;257(22):13550–13556. [PubMed] [Google Scholar]
- Woese C. R., Kandler O., Wheelis M. L. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H. C., Lessie T. G. Branched chain amino acid aminotransferase isoenzymes of Pseudomonas cepacia. Arch Microbiol. 1979 Mar 12;120(3):223–229. doi: 10.1007/BF00423069. [DOI] [PubMed] [Google Scholar]
- Xing R. Y., Whitman W. B. Characterization of enzymes of the branched-chain amino acid biosynthetic pathway in Methanococcus spp. J Bacteriol. 1991 Mar;173(6):2086–2092. doi: 10.1128/jb.173.6.2086-2092.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing R. Y., Whitman W. B. Sulfometuron methyl-sensitive and -resistant acetolactate synthases of the archaebacteria Methanococcus spp. J Bacteriol. 1987 Oct;169(10):4486–4492. doi: 10.1128/jb.169.10.4486-4492.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi T., Kagamiyama H., Nozaki M. Aspartate: 2-oxoglutarate aminotransferase from bakers' yeast: crystallization and characterization. J Biochem. 1982 Jul;92(1):35–43. doi: 10.1093/oxfordjournals.jbchem.a133929. [DOI] [PubMed] [Google Scholar]
- Yagi T., Kagamiyama H., Nozaki M., Soda K. Glutamate-aspartate transaminase from microorganisms. Methods Enzymol. 1985;113:83–89. doi: 10.1016/s0076-6879(85)13020-x. [DOI] [PubMed] [Google Scholar]
- Yagi T., Toyosato M., Soda K. Crystalline aspartate aminotransferase from Pseudomonas striata. FEBS Lett. 1976 Jan 1;61(1):34–37. doi: 10.1016/0014-5793(76)80165-2. [DOI] [PubMed] [Google Scholar]


