Abstract
In Alzheimer disease, β-amyloid peptide accumulates in the brain as insoluble amyloid plaques. Amyloid filaments, similar to those found in amyloid plaques, can be assembled in vitro from chemically synthesized β-peptides. In this study, we report that antibodies raised against the N-terminal region (1–28) of the β-amyloid peptide bind to the in vitro-formed β-amyloid assemblies, leading to disaggregation of the fibrils and partial restoration of the peptide’s solubility. The concomitant addition of fibrillar β-amyloid with these antibodies to PC 12 cells leads to the inhibition of the neurotoxic effects of β-amyloid. Some of the mAbs raised against soluble β-peptide (1–28) have been found to prevent in vitro fibrillar aggregation of β-amyloid peptide. These experimental data suggest that site-directed mAbs interfere with the aggregation of β-amyloid and trigger reversal to its nontoxic, normal components. The above findings give hints on how to convert in vivo senile plaques into nontoxic, diffuse components and may have therapeutic interest for those studying Alzheimer disease and other human diseases related to amyloidogenic properties of physiological peptides and proteins.
One of the major pathological features of Alzheimer disease (AD) is the abundant presence of amyloid plaques in the brain tissues of affected individuals (1). The plaques are predominantly comprised of a 40- to 42-mer β-amyloid peptide (Aβ) whose neurotoxicity is related to the aggregation process (2, 3). In AD, Aβ accumulates in the brain as both diffuse and compact plaques. Diffuse plaques are not associated with degenerative changes whereas compact plaques comprised of Aβ fibrils are associated with pathological changes in the surrounding brain parenchyma (4).
Aβ was found to be a normal metabolite produced during processing of a large transmembrane glycoprotein amyloid protein precursor. Once released by proteolytic cleavage of an amyloid protein precursor, the β-peptide may remain in solution either as a random coil or as an α-helical structure (5, 6). The transition of the α-helix to a β-sheet conformation, with concomitant peptide aggregation, is a proposed mechanism of plaque formation.
The contribution of the C-terminal region of Aβ in the initiation and progression of β-sheet formation has been established (7–9). However, the importance of the N-terminal fragment of the Aβ molecule for fibrillar genesis has only lately been emphasized (10–11). Whereas the hydrophobic segment in the C-terminal domain of Aβ develops a β-strand structure in aqueous solutions, independently of pH or temperature conditions, the N-terminal region can exhibit different conformations and solubility properties depending on environmental conditions (12). Recent studies show that deletion of the 1–12 and 1–17 amino-terminal residues from Aβ accelerates its aggregation in parallel with enhanced neurotoxicity effects (13).
Amyloid filaments, similar to those found in amyloid plaques and cerebrovascular amyloid, can be assembled from chemically synthesized β-peptides under well defined experimental conditions in vitro, and the effect on neural cells may be neurotoxic or neurotrophic, depending on the β-amyloid fibrillar state (14–16).
Recently, we showed that selected mAbs raised against β-peptide interfere with in vitro aggregation of the Aβ, maintaining its solubility under experimental conditions in which the peptide tends to self-aggregate (17). In the present study, we show that site-directed mAbs against various epitopes of the soluble Aβ may selectively disturb the fibrillar, amyloid-like assemblies and inhibit β-amyloid neurotoxicity.
MATERIALS AND METHODS
Synthetic Aβ (1–40) was obtained from k-Biological (Rancho-Cucamonga, CA). In vitro formation of β-amyloid was induced by incubation of an aqueous solution of Aβ (10 mg/ml) for 7 days at 37°C. The extent of β-amyloid formation and disaggregation was monitored using a panel of well characterized mAbs (18–20) raised against soluble Aβ fragments, as follows: mAb 6C6, which recognizes an epitope located in the 1–16 region of Aβ; mAb 14C2 raised against region 13–28 of Aβ; mAb 14C2 raised against residues 33–40 located at the C-terminal region of the Aβ; and CP4 raised against carboxypeptidase A prepared in our laboratory and used as an unrelated antibody as control. The anti-Aβ mAbs were provided by D. Schenk (Athena Neuroscience, San Francisco).
Cell Culture and Cytotoxicity Assay.
Rat pheochromocytoma PC 12 cells were cultured in DMEM supplemented with 5% horse serum, 10% fetal calf serum, 2 mM l-glutamine, and 100 units/ml penicillin/streptomycin and incubated at 37°C under 5% CO2.
For the neurotoxicity assay, cultured PC 12 cells were seeded into a 96-well plate at a density of 104 cells/100 μl/well in a serum-free medium supplemented with 2 μM of insulin. The dose-dependent neurotoxicity was measured using samples of fibrillar β-amyloid obtained after 7-day aging at 37°C of an aqueous solution of Aβ (250 μM). The β-amyloid, at concentrations ranging between 0.025 and 25 μM, was added to the wells containing PC 12 cells. The plates were incubated at 37°C for 2 days, after which cell viability was assessed by measuring cellular redox activity with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), as described (21–24). MTT was added to the wells at a final concentration of 1 mg/ml and incubated with the cells for a further 3 h at 37°C. Cell lysis buffer (20% wt/vol SDS in a solution of 50% dimethylformamide, pH 7.4) was added, and the plate was incubated overnight at 37°C. MTT reduction was determined colorimetrically by the change in OD at 550 nm using an automated microplate spectrophotometer. The effect of mAbs on the inhibition of neurotoxicity of β-amyloid was measured as follows: In a first set of experiments, fibrillar β-amyloid (0.25 μM), 100 μl, was added concomitantly with increasing concentrations of the studied antibodies, e.g., 6C6, 14C2, 1C2, and CP4 (at molar ratios of 100 and 10:1 Aβ/mAb) in a constant volume of 150 μl to the wells containing PC 12, and cell viability was measured. In another set of experiments, samples of the fibrillar β-amyloid (2.5 μM) (previously incubated with mAb 6C6 and/or with the unrelated antibody for 24 h at a molar ratio of 10:1 Aβ/mAb) were added to the wells containing the PC 12 cells, and the cell viability was measured as described above. The assay values (an average of triplicates obtained for each well) were related to the numbers obtained in the absence of the β-amyloid, considered as 100% viability.
Formation and Disaggregation of β-Amyloid.
Reaction mixture tubes (previously coated with nonfat milk to avoid nonspecific binding) containing aliquots of 100 ng of an aqueous stock solution of Aβ (10 mg/ml) were incubated at 37°C for 7 days. The soluble and aggregated forms of Aβ were separated by centrifugation at 12,000 × g for 15 min in an Eppendorf microfuge at 25°C. The β-amyloid aggregates were washed several times with 25 μl of PBS. The first supernatant, containing the residual soluble Aβ, and the washing solutions were collected and incubated for 60 min with excess of mAb 6C6 to produce immunocomplexed Aβ, which was estimated using a sandwich ELISA (17). After removal of the residual soluble Aβ, 2 μg/100 μl PBS of each of the studied mAbs were added to the washed β-amyloid, and the mixtures were reincubated at 37°C for 1 more day. The equimolar ratio mAb/Aβ required for maximum protection effect against peptide aggregation previously established (25) was confirmed by the recent experiments. The reaction mixtures were separated by centrifugation from the insoluble β-amyloid under the same experimental conditions described above. The residual β-amyloid was washed twice with 25 μl of PBS by centrifugation under the same conditions. The washing solutions collected were added to the first supernatants containing the resolubilized β-amyloid–mAb complexes. The amounts of resolubilized β-amyloid–mAb complexes were measured by ELISA using polyclonal anti-β-peptide antibodies as coating antibodies (Boehringer Mannheim).
Thioflavin T (ThT) Fluorimetry.
Aggregation of Aβ was measured by the ThT binding assay, in which the fluorescence intensity reflects the degree of β-amyloid fibrillar aggregation. ThT characteristically stains amyloid-like deposits (26) and exhibits enhanced fluorescence emission of 482 nm and a new excitation peak of 450 nm when added to a suspension of aggregated β-sheet preparations.
Aqueous solutions of Aβ samples in PBS (30 μg/10 μl) were incubated at 37°C for 1 week. Various mAbs were added to the preformed fibrils of β-amyloid at differing molar ratios, and the reaction mixtures were incubated for 1 day at 37°C. The fluorescence was measured after addition of 1 ml of ThT (3 μM in 50 mM sodium phosphate buffer, pH 6.0).
RESULTS
Epitope-Specific Effect of mAbs on the Solubilization of β-Amyloid.
Exposure of Aβ to 37°C for 1 week under the experimental conditions used led to fibrillar formation of β-amyloid, leaving less than 5% soluble peptide. The fibrillar nature of the aggregates has been characterized by electron microscopy and ThT fluorescence studies (17).
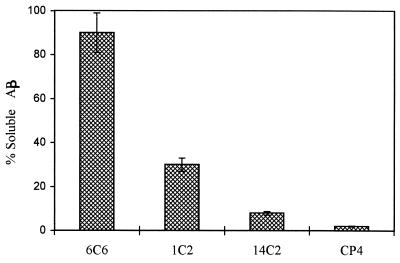
To determine whether the effect of mAbs on the solubilization of β-amyloid is a function of a specific Aβ domain or a more general result of interaction with a high molecular weight molecule such as an antibody, a panel of mAbs directed to different regions of the Aβ was incubated with 1-week-old β-amyloid. The equimolar ratio Aβ/Ab used in this experiment was based on data previously obtained for the maximal protection effect of mAbs against protein aggregation, as recently reported (25, 27). Under experimental conditions used, mAb 6C6, which binds to an epitope located in the 1–16 region, had a significant solubilization effect on β-amyloid, ranging between 80 and 98%, after 1 day of incubation at 37°C with the preformed fibrillar β-amyloid (Fig. 1). mAb 1C2, which binds to region 13–28 of the Aβ, was considerably less effective in restoring the solubility of the peptide. mAb 14C2, raised against the C-terminal region, had no sensitive effect on Aβ solubility similar to the unrelated mAb CP4.
Figure 1.
Solubilization of β-amyloid in the presence of mAbs 6C6, 1C2 and 14C2. The amounts of immunocomplexes of the resolubilized Aβ obtained after 24-h incubation with the respective antibodies were measured by a sandwich ELISA assay using rabbit polyclonal antibodies raised against Aβ as coating antibodies. Measurement of the OD of A495 monitors antibody binding to β-amyloid using horseradish peroxidase-labeled goat anti-mouse antibody (Bio-Rad). The data represent the mean of three replicates. The SDs of the intraassay and interassay were 8%. The percentages are related to maximal OD measured for each mAb after the addition of 100 ng of soluble Aβ complexed with each of the studied mAb to the coated ELISA wells before incubation at 37°C.
mAbs Disrupt Fibrillar Structure of β-Amyloid.
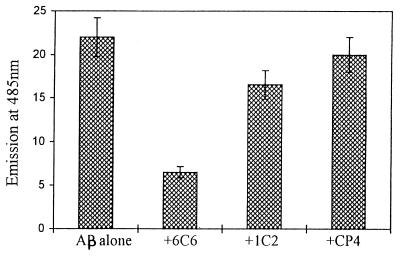
Fibril formation was achieved by incubation of the aliquots of Aβ for 1 week and was quantitated by ThT fluorometry binding assay. After 1 week of incubation at 37°C, mAbs 6C6 and 1C2, as well as antibodies raised against unrelated proteins at various molar ratios, were added to the already formed fibrillar amyloid, and the mixtures were reincubated at 37°C for 1 more day. The antibody 6C6, at an antibody concentration of 10:1 molar ratio Aβ/mAb, disrupted the fibrillar structure of β-amyloid with extensive deterioration of fibril morphology, as indicated by a substantial decrease in ThT fluorescence (Fig. 2). However, an equimolar ratio (mAb/Aβ) of mAb 6C6 was required to reach the maximum solubilization effect of β-amyloid fibrils. mAb 1C2, which binds to an epitope located on the region 13–28 (assumed to be in the interior site of the hydrophobic core), interfered only slightly with fibril disaggregation. The disaggregation of the fibrils required a relatively short incubation time with the antibodies, and longer exposure (1 week) had an insignificantly increased effect on the β-amyloid disaggregation (data not shown). The decrease in ThT fluorescence intensity occurred in the presence of a low ratio mAb/Aβ (1:10), suggesting the deterioration of the fibrillar aggregate into amorphous structures, which proved to be nontoxic to PC 12 cells.
Figure 2.
ThT-based fluorometric assay. Estimation of the fluorescence of ThT, which correlates with the amount of fibrillar β-amyloid formed after incubation for 1 week at 37°C before and after additional incubation for 24 h with mAbs 6C6 and 1C2 and unrelated antibodies (at molar ratio 10:1 Aβ/mAb) using the ThT assay as described.
Neurotoxicity Detection.
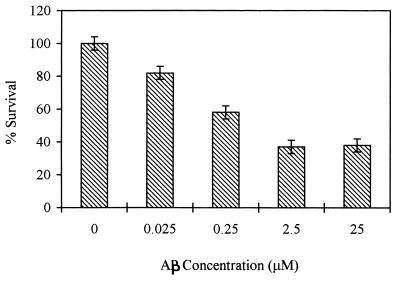
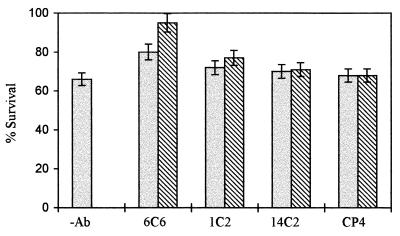
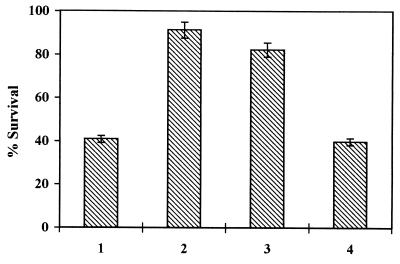
In vitro neurotoxicity of Aβ to rat pheochromocytoma PC 12 cells was measured after incubating the cells for 2 days with increasing concentrations of the aggregated β-peptide. Viability of the cells exposed to β-amyloid was assessed by their ability to reduce MTT using a mitochondrial enzyme generating formazan. As shown in Fig. 3, Aβ neurotoxicity was dose-dependent in the range of 0.025–25 μM, and cell survival (expressed as the percentage of control measured in the absence of β-amyloid) decreased to 37%. Concomitant addition of β-amyloid with mAb 6C6 at molar ratios ranging from 10 to 100 Aβ/mAb prevented the neurotoxicity of the β-amyloid in a concentration-dependent manner (Fig. 4) whereas other studied antibodies exhibited an insignificant protection effect. The effects of mAbs in preventing neurotoxicity were assessed also by adding either the β-amyloid previously incubated with the mAbs or the soluble β-peptide incubated for 7 days at 37°C in the presence of the mAbs to the cell cultures (molar ratio mAb/Aβ 1:10) (Fig. 5). The results show that, in both figures, mAb 6C6 had a protective effect on PC 12 cells; neurotoxicity of β-amyloid was prevented either when mAb 6C6 was added to the Aβ before the aggregation process or when the antibodies were added for another 24 h to β-amyloid already formed after 7 days at 37°C.
Figure 3.
Dose-dependent neurotoxicity of β-amyloid aggregates on PC 12 cells measured by MTT assay. Increasing concentrations of β-amyloid aged 1 week were added to the same amount of cultured cells and assayed as described.
Figure 4.
Selective inhibitory effect of various mAbs on the β-amyloid toxicity toward PC 12 cells using the MTT assay. The mAbs were added to 0.25 μM β-amyloid at molar ratios of 100:1 Aβ/mAb (left bars) and 10:1 Aβ/mAb (right bars), and the mixtures were added to the cells. The percentages are related to cell viability measured in the absence of the β-amyloid, which is considered 100%.
Figure 5.
The effect of mAb 6C6 on the inhibition of fibrillar β-amyloid neurotoxicity toward PC12 cells using the MTT assay. The following preparations were added to the cells for 48 h: (i) Aβ (2.5 μM) preincubated for 7 days at 37°C; (ii) soluble Aβ (2.5 μM) incubated for 7 days at 37°C in the presence of mAb 6C6 (molar ratio mAb/Aβ 1:10); (iii) preformed β-amyloid (2.5 μM) incubated for a further 24 h with mAb 6C6 (molar ratio mAb/Aβ 1:10); and (iv) Aβ incubated with unrelated antibodies. The percentages are related to the cell viability measured in the absence of β-amyloid, which is considered as 100%.
DISCUSSION
Antibody–antigen interactions involve conformational changes in both antibody and antigen that can range from insignificant to considerable (28–30). Binding of high affinity mAbs to regions of high flexibility and antigenicity may alter the molecular dynamics of the whole protein chain or assembly (31, 32). mAbs were found to be able to recognize incompletely folded epitopes and to induce native conformation in partially or wrongly folded protein (33–35).
In the present study, we show that site-directed mAbs toward N-terminal regions of the β-peptide can bind to preformed β-amyloid fibrils, leading to their disaggregation. The prevention of peptide aggregation, as well as the solubilization of already formed aggregates, required an equimolar ratio of mAb/peptide, indicating the molecular level of these interactions (17, 25, 27). Binding of high affinity mAbs against the N-terminal region of Aβ at lower antibody concentrations interfered with noncovalent interactions between the fibrils and lead to disaggregation and deterioration of assembly of amyloid fibrils. The deterioration of assembly of amyloid fibrils was found to be accompanied by inhibition of the neurotoxicity effect of β-amyloid. The disaggregation effect was found to be dependent on the location of the epitopes on the β-amyloid and the binding characteristics of the mAbs. The N-terminal region of the β-peptide was suggested to be the immunodominant site in Aβ. mAbs raised against Aβ fragments comprising amino acids 1–16 show that this region exhibits increased antigenicity characteristics compared with the rest of the β-peptides (B. Solomon, personal communication). Recent studies on antibody recognition of synthetic Aβ showed that antiserum raised against either native β-amyloid or synthetic β-peptide 1–28 is highly reactive with β-peptide 6–20 and 1–38 but not with β-peptide 22–35 (36).
Formation of β-amyloid fibers involves a number of steps, including the folding and association of peptide monomers via hydrogen bonding and intersheet packing to create a β-crystallite subunit from which the fibril can be assembled (37). Strong interactions occurring at the C-terminal result in self-association and formation of an anti-parallel, β-fibrillar core. Interactions involving the N-terminal region suggest that at least the residues 1–9 of Aβ are not necessary for filament formation (38). Localization of the Aβ N terminus to the exterior of the molecule is supported by the Soreghan et al. (39) β-amyloid fibril model, which leaves this region accessible to bind other proteins. The N-terminal region seems to provide the means for interfibrillary contacts, as well as for interactions between a filament and other proteins or cellular structures that are often associated with β-amyloid depositions in vivo. As recently reported (40), this region contains an anti-chymotrypsin binding site and, under certain experimental conditions, in its presence, the preformed β-amyloid undergoes a morphological transition to partially disaggregated fibrils.
Binding of mAbs directed against the N-terminal region leads to inhibition of the neurotoxicity effect. Several studies suggest that the neurotoxicity effect correlates with the formation of Aβ aggregates and with the extent of the β-sheet structure (2, 3, 13, 22, 23). The effects of Aβ on MTT reduction on PC 12 cells are known to occur at concentrations below those that result in cell death (24) and represent early markers of the metabolic compromise that ultimately leads to cellular degeneration. After binding of mAb 6C6 to fibrillar β-amyloid, the neurotoxicity of Aβ, as measured by MTT assay, is prevented because of disaggregation of β-amyloid fibrils. A slight inhibition effect was obtained after addition of other amyloid peptide antibodies to PC 12 cells.
These findings suggest that high affinity, site-directed mAbs (or compounds that may mimic their biological activities as genetically engineered small antibodies or peptide mimetics) trigger reversal of the pathological aggregation of β-amyloid to its nontoxic components. Several human diseases appear to be related to amyloidogenic peptides and proteins (41–44). Aggregation mechanisms, very similar to those of Aβ, are suggested for prion proteins. Development of such compounds may develop a new class of active molecules in AD and/or prion disease therapy.
Acknowledgments
We thank Dr. D. Schenk and Ms. R. Motter (Athena Neuroscience, San Francisco) for generosity in supplying mAbs and Mrs. F. Margolin for manuscript editing.
ABBREVIATIONS
- AD
Alzheimer disease
- Aβ
β-amyloid peptide
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- ThT
thioflavin T
References
- 1.Selkoe D J. Trends Neurochem Sci. 1993;16:403–409. doi: 10.1016/0166-2236(93)90008-a. [DOI] [PubMed] [Google Scholar]
- 2.Pike C J, Burdick D, Walencewics A J, Glabe C G, Cotman C W. J Neurosci. 1993;13:1676–1687. doi: 10.1523/JNEUROSCI.13-04-01676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pike C J, Cotman C W. Brain Res. 1995;671:293–298. doi: 10.1016/0006-8993(94)01354-k. [DOI] [PubMed] [Google Scholar]
- 4.Lorenzo A, Yankner B A. Proc Natl Acad Sci USA. 1994;91:12243–12247. doi: 10.1073/pnas.91.25.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, Sinha S, Schlossmacher M, Whaley J, Swindlehurst C, McCormack R, Wolfert R, Selkoe D, Lieberburg I, Schenk D. Nature (London) 1992;359:325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- 6.Shoji M, Golde T E, Ghiso J, Cheung T T, Estus S, Shaffer L M, Cai X-D, McKay D M, Tintner R, Frangione B, Younkin S G. Science. 1992;258:126–129. doi: 10.1126/science.1439760. [DOI] [PubMed] [Google Scholar]
- 7.Halverson K, Fraser P, Kirschner D A, Lansbury P T., Jr Biochemistry. 1990;29:2639–2644. doi: 10.1021/bi00463a003. [DOI] [PubMed] [Google Scholar]
- 8.Hilbich C, Kisters-Woike B, Reed J, Masters C L, Beyreuther K. J Mol Biol. 1991;218:149–163. doi: 10.1016/0022-2836(91)90881-6. [DOI] [PubMed] [Google Scholar]
- 9.Jarrett J T, Lansbury P T., Jr Cell. 1993;73:1055–1056. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- 10.Saido T C, Iwatsubo T, Mann D M A, Shimada H, Ihara Y, Kawashima S. Neuron. 1995;14:457–466. doi: 10.1016/0896-6273(95)90301-1. [DOI] [PubMed] [Google Scholar]
- 11.Gowing E, Roher A E, Woods A S, Cotter R J, Chaney M, Little S P, Ball M J. J Biol Chem. 1994;269:10987–10990. [PubMed] [Google Scholar]
- 12.Barrow C J, Zagorski M R. Science. 1991;253:179–182. doi: 10.1126/science.1853202. [DOI] [PubMed] [Google Scholar]
- 13.Pike C J, Overman M J, Cotman C W. J Biol Chem. 1995;270:23895–23898. doi: 10.1074/jbc.270.41.23895. [DOI] [PubMed] [Google Scholar]
- 14.Yankner B A, Duffy L K, Kirschner D A. Science. 1990;250:279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]
- 15.Howlett D R, Jennings K H, Lee D C, Clark M S G, Brown F, Wetzel R, Wood S J, Camilleri P, Roberts G W. Neurodegeneration. 1995;4:23–32. doi: 10.1006/neur.1995.0003. [DOI] [PubMed] [Google Scholar]
- 16.Hasegawa Y, Sugimoto E, Endo T, Ogawa K, Aratake H, Morikawa A, Kitaguchi N. Biol Pharmacol Bull. 1995;18:1750–1754. doi: 10.1248/bpb.18.1750. [DOI] [PubMed] [Google Scholar]
- 17.Solomon B, Koppel R, Hanan E, Katzav T. Proc Natl Acad Sci USA. 1996;93:452–455. doi: 10.1073/pnas.93.1.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esch F S, Keim P S, Beattie E C, Blacher R W, Culwell A R, Oltersdorf T, McClure D, Ward P J. Science. 1990;248:1122–1124. doi: 10.1126/science.2111583. [DOI] [PubMed] [Google Scholar]
- 19.Haass S C, Schlossmacher M G, Hung A Y, Vigo- Pelfrey C, Mellon A, Ostaszewski B L, Lieberburg I, Koo E H, Schenk D, Teplow D B, Selkoe D J. Nature (London) 1992;359:322–327. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- 20.Hyman B T, Tanzi R B, Marzloff K, Barbour R, Schenk D B. J Neuropathol Exp Neurol. 1992;51:76–83. doi: 10.1097/00005072-199201000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Hansen M B, Nielsen S E, Berg K. J Immunol Methods. 1989;119:203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- 22.Shearman M S, Ragan C I, Iversen L L. Proc Natl Acad Sci USA. 1994;91:1470–1474. doi: 10.1073/pnas.91.4.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomiyama T, Asano S, Suwa Y, Morita T, Kataoka K-I, Mori H, Endo N. Biochem Biophys Res Commun. 1994;204:76–83. doi: 10.1006/bbrc.1994.2428. [DOI] [PubMed] [Google Scholar]
- 24.Shearman M S, Hawtin S R, Tailor V J. J Neurochem. 1995;65:218–227. doi: 10.1046/j.1471-4159.1995.65010218.x. [DOI] [PubMed] [Google Scholar]
- 25.Katzav-Gozansky T, Hanan E, Solomon B. Biotechnol Appl Biochem. 1996;23:227–230. [PubMed] [Google Scholar]
- 26.LeVine H., III Protein Sci. 1993;2:404–410. doi: 10.1002/pro.5560020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanan E, Solomon B. Amyloid Int J Exp Clin Invest. 1996;3:130–133. [Google Scholar]
- 28.Davies D R, Cohen G H. Proc Natl Acad Sci USA. 1996;93:7–12. doi: 10.1073/pnas.93.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amit A G, Mariuzza R A, Phillips S E V, Poljak R J. Science. 1986;233:747–753. doi: 10.1126/science.2426778. [DOI] [PubMed] [Google Scholar]
- 30.Bhat T N, Bentley G A, Fischmann T O, Boulot G, Poljak R J. Nature (London) 1990;347:483–485. doi: 10.1038/347483a0. [DOI] [PubMed] [Google Scholar]
- 31.Frauenfelder H, Petsko G A, Tsernoglou D. Nature (London) 1979;280:558–563. doi: 10.1038/280558a0. [DOI] [PubMed] [Google Scholar]
- 32.Karplus M, Petsko G A. Nature (London) 1990;347:631– 639. doi: 10.1038/347631a0. [DOI] [PubMed] [Google Scholar]
- 33.Blond S, Goldberg M. Proc Natl Acad Sci USA. 1987;84:1147–1151. doi: 10.1073/pnas.84.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carlson J D, Yarmush M L. Bio/Technology. 1992;10:86–91. doi: 10.1038/nbt0192-86. [DOI] [PubMed] [Google Scholar]
- 35.Solomon B, Schwartz F. J Mol Recognit. 1995;8:72–76. doi: 10.1002/jmr.300080113. [DOI] [PubMed] [Google Scholar]
- 36.Fraser P E, Duffy L K, O’Malley M B, Nguyen J, Inouye H, Kirschner D A. J Neurosci Res. 1991;28:474–485. doi: 10.1002/jnr.490280404. [DOI] [PubMed] [Google Scholar]
- 37.Inouye H, Fraser P E, Kirschner D A. Biophys J. 1993;64:502–519. doi: 10.1016/S0006-3495(93)81393-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirschner D A, Inouye H, Duffy L K, Sinclair A, Lind M, Selkoe D J. Proc Natl Acad Sci USA. 1987;84:6953–6957. doi: 10.1073/pnas.84.19.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soreghan B, Kosmoski J, Glabe C. J Biol Chem. 1994;269:28551–28554. [PubMed] [Google Scholar]
- 40.Fraser P E, Nguyen J T, McLachlan D R, Abraham C R, Kirschner D A. J Neurochem. 1993;61:298–305. doi: 10.1111/j.1471-4159.1993.tb03568.x. [DOI] [PubMed] [Google Scholar]
- 41.Goldfarb L G, Brown P, Haltia M, Ghiso J, Frangione B, Gajdusek D C. Proc Natl Acad Sci USA. 1993;90:4451–4454. doi: 10.1073/pnas.90.10.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.May P C, Boggs L N, Fuson K S. J Neurochem. 1993;61:2330–2333. doi: 10.1111/j.1471-4159.1993.tb07480.x. [DOI] [PubMed] [Google Scholar]
- 43.Forloni G, Angeretti N, Chiesa R, Monzani E, Salmona M, Bugiani O, Tagliavini F. Nature (London) 1993;362:543–546. doi: 10.1038/362543a0. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen J, Baldwin M A, Cohen F E, Prusiner S B. Biochemistry. 1995;34:4186–4192. doi: 10.1021/bi00013a006. [DOI] [PubMed] [Google Scholar]