Abstract
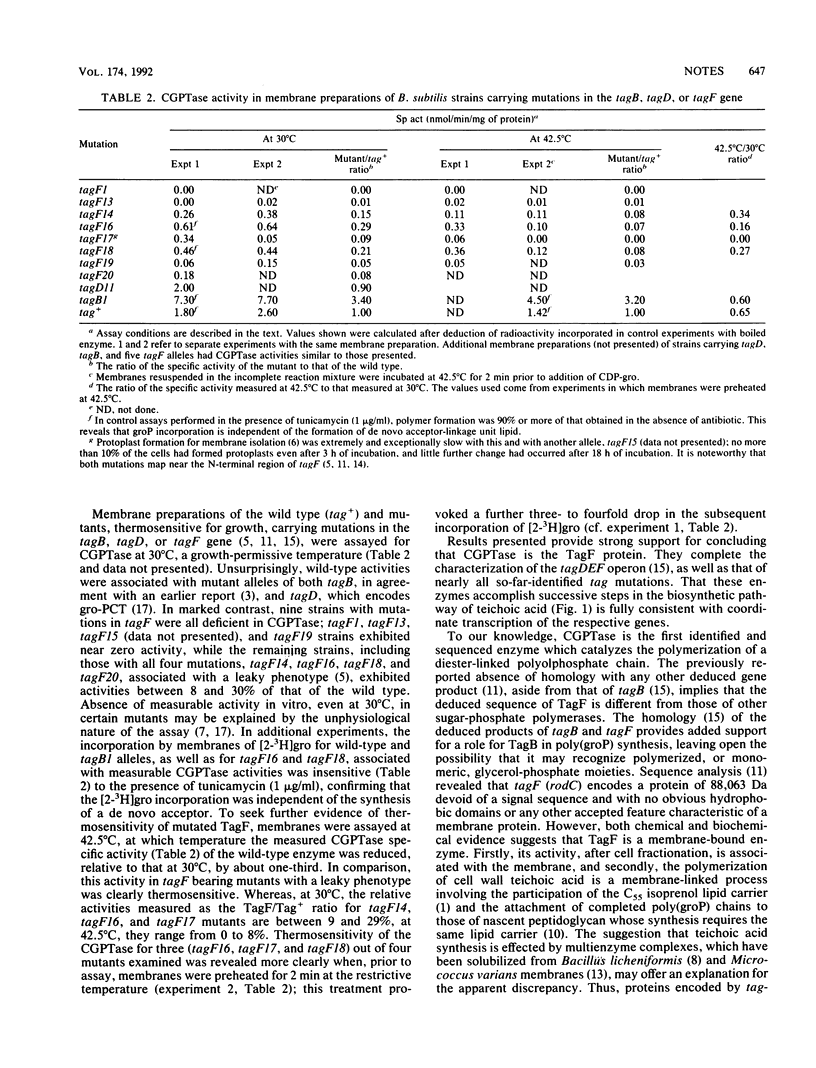
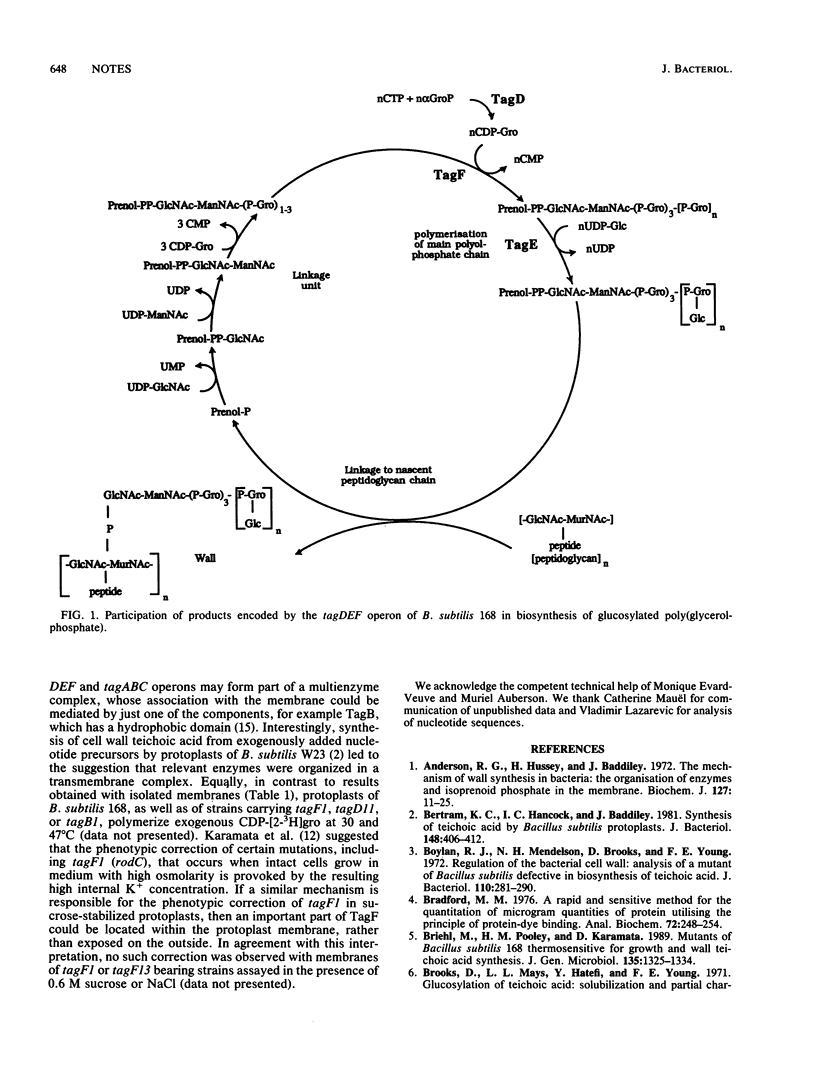
Assays of CDP-glycerol:poly(glycerophosphate) glycerophosphotransferase (CGPTase) (EC 2.7.8.12) in membranes isolated from Bacillus subtilis 168 wild type and 11 strains bearing conditional lethal thermosensitive mutations in tagB, tagD, or tagF revealed that CGPTase deficiency was associated only with mutant tagF alleles. In vitro, thermosensitivity of CGPTase strongly suggests that the structural gene for this enzyme is tagF. We discuss apparent discrepancies between biochemical evidence favoring a membrane location for TagF and a previous report that suggested a cytoplasmic location based on sequence analysis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertram K. C., Hancock I. C., Baddiley J. Synthesis of teichoic acid by Bacillus subtilis protoplasts. J Bacteriol. 1981 Nov;148(2):406–412. doi: 10.1128/jb.148.2.406-412.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan R. J., Mendelson N. H., Brooks D., Young F. E. Regulation of the bacterial cell wall: analysis of a mutant of Bacillus subtilis defective in biosynthesis of teichoic acid. J Bacteriol. 1972 Apr;110(1):281–290. doi: 10.1128/jb.110.1.281-290.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Estrela A. I., Pooley H. M., de Lencastre H., Karamata D. Genetic and biochemical characterization of Bacillus subtilis 168 mutants specifically blocked in the synthesis of the teichoic acid poly(3-O-beta-D-glucopyranosyl-N-acetylgalactosamine 1-phosphate): gneA, a new locus, is associated with UDP-N-acetylglucosamine 4-epimerase activity. J Gen Microbiol. 1991 Apr;137(4):943–950. doi: 10.1099/00221287-137-4-943. [DOI] [PubMed] [Google Scholar]
- Hancock I. C., Baddiley J. Solubilisation of a teichoic acid-synthesising system from the membrane of Bacillus licheniformis by freezing and thawing. FEBS Lett. 1973 Aug 1;34(1):15–18. doi: 10.1016/0014-5793(73)80692-1. [DOI] [PubMed] [Google Scholar]
- Hancock I. C., Wiseman G., Baddiley J. Biosynthesis of the unit that links teichoic acid to the bacterial wall: inhibition by tunicamycin. FEBS Lett. 1976 Oct 15;69(1):75–80. doi: 10.1016/0014-5793(76)80657-6. [DOI] [PubMed] [Google Scholar]
- Higashi Y., Strominger J. L., Sweeley C. C. Biosynthesis of the peptidoglycan of bacterial cell walls. XXI. Isolation of free C55-isoprenoid alcohol and of lipid intermediates in peptidoglycan synthesis from Staphylococcus aureus. J Biol Chem. 1970 Jul 25;245(14):3697–3702. [PubMed] [Google Scholar]
- Honeyman A. L., Stewart G. C. The nucleotide sequence of the rodC operon of Bacillus subtilis. Mol Microbiol. 1989 Sep;3(9):1257–1268. doi: 10.1111/j.1365-2958.1989.tb00276.x. [DOI] [PubMed] [Google Scholar]
- Karamata D., McConnell M., Rogers H. J. Mapping of rod mutants of Bacillus subtilis. J Bacteriol. 1972 Jul;111(1):73–79. doi: 10.1128/jb.111.1.73-79.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver J., Hancock I. C., Baddiley J. Fractionation studies of the enzyme complex involved in teichoic acid synthesis. J Bacteriol. 1981 Jun;146(3):847–852. doi: 10.1128/jb.146.3.847-852.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauël C., Young M., Karamata D. Genes concerned with synthesis of poly(glycerol phosphate), the essential teichoic acid in Bacillus subtilis strain 168, are organized in two divergent transcription units. J Gen Microbiol. 1991 Apr;137(4):929–941. doi: 10.1099/00221287-137-4-929. [DOI] [PubMed] [Google Scholar]
- Mauël C., Young M., Margot P., Karamata D. The essential nature of teichoic acids in Bacillus subtilis as revealed by insertional mutagenesis. Mol Gen Genet. 1989 Feb;215(3):388–394. doi: 10.1007/BF00427034. [DOI] [PubMed] [Google Scholar]
- Pooley H. M., Abellan F. X., Karamata D. A conditional-lethal mutant of bacillus subtilis 168 with a thermosensitive glycerol-3-phosphate cytidylyltransferase, an enzyme specific for the synthesis of the major wall teichoic acid. J Gen Microbiol. 1991 Apr;137(4):921–928. doi: 10.1099/00221287-137-4-921. [DOI] [PubMed] [Google Scholar]
- Pooley H. M., Paschoud D., Karamata D. The gtaB marker in Bacillus subtilis 168 is associated with a deficiency in UDPglucose pyrophosphorylase. J Gen Microbiol. 1987 Dec;133(12):3481–3493. doi: 10.1099/00221287-133-12-3481. [DOI] [PubMed] [Google Scholar]
- Ward J. B., Wyke A. W., Curtis C. A. The effect of tunicamycin on wall-polymer synthesis in Bacilli. Biochem Soc Trans. 1980 Apr;8(2):164–166. doi: 10.1042/bst0080164. [DOI] [PubMed] [Google Scholar]


