Abstract
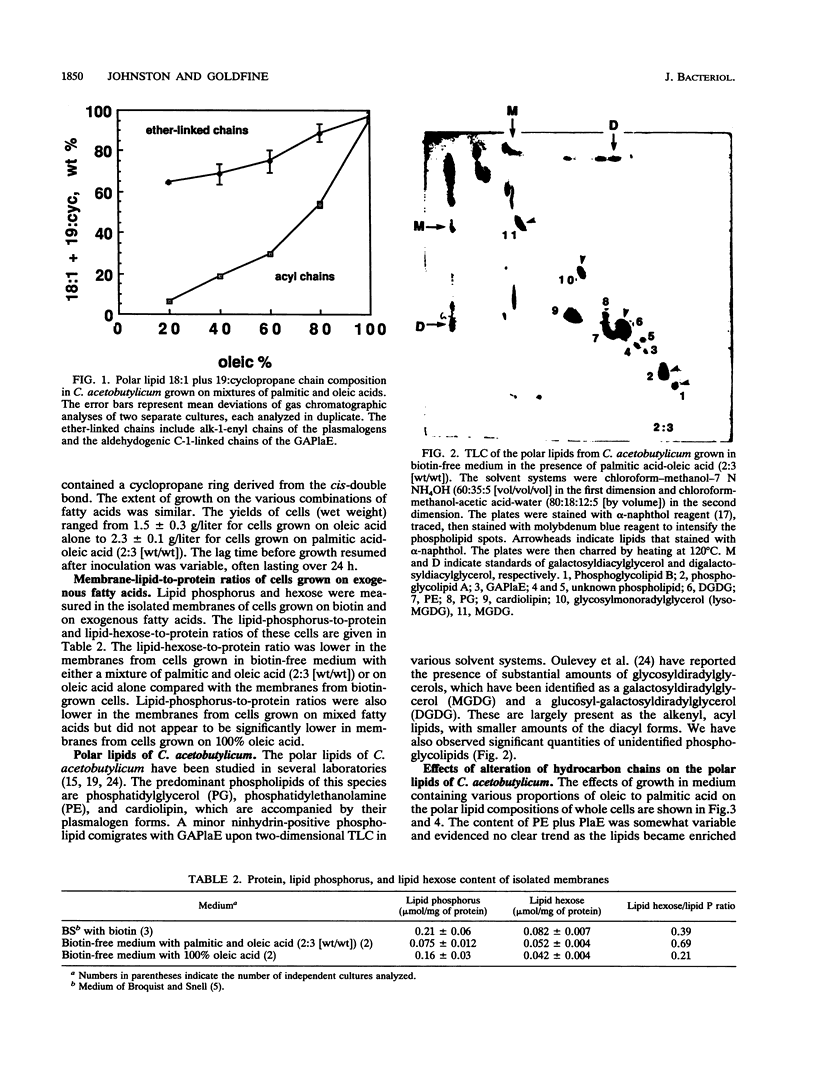
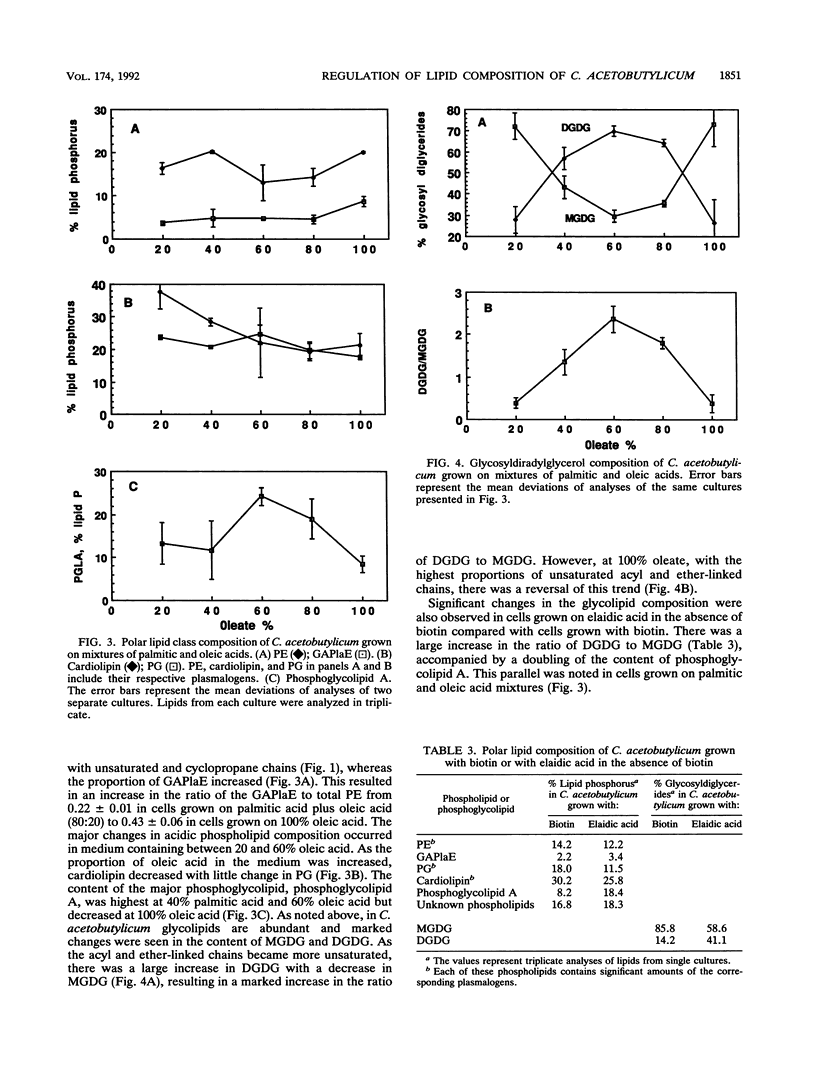
The membrane lipid aliphatic chains of Clostridium acetobutylicum ATCC 4259 have been extensively modified by growth in biotin-free medium containing vitamin-free casein hydrolysate supplemented with either elaidic acid, oleic acid, or mixtures of palmitic and oleic acids. Growth with elaidic acid resulted in polar lipids containing 88.6% 18:1 acyl chains and 94.5% 18:1 ether-linked chains. Growth with oleic acid resulted in comparable levels of enrichment of the lipids with 18:1 chains and C19 chains containing cyclopropane rings. When cells were grown with mixtures of palmitic and oleic acids, the ether-linked chains of the plasmalogens were greater than or equal to 64% 18:1 plus C19 chains containing cyclopropane rings at all ratios of oleic to palmitic acid in the medium. The acyl chains reflected the palmitic acid content of the medium more closely. Marked changes were observed in both phospholipid and glycosyldiglyceride compositions as the lipid acyl and ether-linked chains became more enriched with unsaturated and cyclopropane chains. The ratio of the glycerol acetal of plasmenylethanolamine to phosphatidylethanolamine increased, the ratio of cardiolipin to phosphatidylglycerol decreased, and the ratio of diglycosyldiglyceride to monoglycosyldiglyceride increased. However, the monoglycosyldiglyceride/diglycosyldiglyceride ratio was lower for cells grown on 100% oleic acid than for cells grown on 60 or 80% oleic acid. In the membranes of cells grown on 100% oleic acid, the ratio of glycolipids to phospholipids was lower than that found in cells grown on 60% oleic acid. These results indicate that C. acetobutylicum regulates its polar lipid composition in a complex manner involving phospholipids and glycosyldiglycerides. These changes can affect the equilibria between those lipids that form bilayers and those lipids that tend to form nonlamellar phases when enriched with unsaturated aliphatic chains. Phosphoglycolipids of unknown structure were also observed in cells grown either with biotin or with fatty acids. The content of the most abundant phosphoglycolipid also varied with the degree of unsaturation of the cellular lipids.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- BROQUIST H. P., SNELL E. E. Biotin and bacterial growth. I. Relation to aspartate, oleate, and carbon dioxide. J Biol Chem. 1951 Jan;188(1):431–444. [PubMed] [Google Scholar]
- Bittman R., Clejan S., Hui S. W. Increased rates of lipid exchange between Mycoplasma capricolum membranes and vesicles in relation to the propensity of forming nonbilayer lipid structures. J Biol Chem. 1990 Sep 5;265(25):15110–15117. [PubMed] [Google Scholar]
- GOLDFINE H., BLOCH K. On the origin of unsaturated fatty acids in clostridia. J Biol Chem. 1961 Oct;236:2596–2601. [PubMed] [Google Scholar]
- Goldfine H. Bacterial membranes and lipid packing theory. J Lipid Res. 1984 Dec 15;25(13):1501–1507. [PubMed] [Google Scholar]
- Goldfine H., Johnston N. C., Mattai J., Shipley G. G. Regulation of bilayer stability in Clostridium butyricum: studies on the polymorphic phase behavior of the ether lipids. Biochemistry. 1987 May 19;26(10):2814–2822. doi: 10.1021/bi00384a024. [DOI] [PubMed] [Google Scholar]
- Goldfine H., Johnston N. C., Phillips M. C. Phase behavior of ether lipids from Clostridium butyricum. Biochemistry. 1981 May 12;20(10):2908–2916. doi: 10.1021/bi00513a030. [DOI] [PubMed] [Google Scholar]
- Goldfine H., Rosenthal J. J., Johnston N. C. Lipid shape as a determinant of lipid composition in Clostridium butyricum. The effects of incorporation of various fatty acids on the ratios of the major ether lipids. Biochim Biophys Acta. 1987 Nov 13;904(2):283–289. doi: 10.1016/0005-2736(87)90377-4. [DOI] [PubMed] [Google Scholar]
- Gruner S. M. Intrinsic curvature hypothesis for biomembrane lipid composition: a role for nonbilayer lipids. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3665–3669. doi: 10.1073/pnas.82.11.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston N. C., Goldfine H. Lipid composition in the classification of the butyric acid-producing clostridia. J Gen Microbiol. 1983 Apr;129(4):1075–1081. doi: 10.1099/00221287-129-4-1075. [DOI] [PubMed] [Google Scholar]
- Johnston N. C., Goldfine H. Phospholipid aliphatic chain composition modulates lipid class composition, but not lipid asymmetry in Clostridium butyricum. Biochim Biophys Acta. 1985 Feb 28;813(1):10–18. doi: 10.1016/0005-2736(85)90339-6. [DOI] [PubMed] [Google Scholar]
- Jones D. T., Woods D. R. Acetone-butanol fermentation revisited. Microbiol Rev. 1986 Dec;50(4):484–524. doi: 10.1128/mr.50.4.484-524.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khuller G. K., Goldfine H. Replacement of acyl and alk-1-enyl groups in Clostridium butyricum phospholipids by exogenous fatty acids. Biochemistry. 1975 Aug 12;14(16):3642–3647. doi: 10.1021/bi00687a020. [DOI] [PubMed] [Google Scholar]
- MacDonald D. L., Goldfine H. Effects of solvents and alcohols on the polar lipid composition of Clostridium butyricum under conditions of controlled lipid chain composition. Appl Environ Microbiol. 1991 Dec;57(12):3517–3521. doi: 10.1128/aem.57.12.3517-3521.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon J. M. Structure of the inverted hexagonal (HII) phase, and non-lamellar phase transitions of lipids. Biochim Biophys Acta. 1990 Feb 28;1031(1):1–69. doi: 10.1016/0304-4157(90)90002-t. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- WEISS S. B., KENNEDY E. P., KIYASU J. Y. The enzymatic synthesis of triglycerides. J Biol Chem. 1960 Jan;235:40–44. [PubMed] [Google Scholar]
- Wieslander A., Christiansson A., Rilfors L., Lindblom G. Lipid bilayer stability in membranes. Regulation of lipid composition in Acholeplasma laidlawii as governed by molecular shape. Biochemistry. 1980 Aug 5;19(16):3650–3655. doi: 10.1021/bi00557a002. [DOI] [PubMed] [Google Scholar]
- Wieslander A., Rilfors L., Johansson L. B., Lindblom G. Reversed cubic phase with membrane glucolipids from Acholeplasma laidlawii. 1H, 2H, and diffusion nuclear magnetic resonance measurements. Biochemistry. 1981 Feb 17;20(4):730–735. doi: 10.1021/bi00507a010. [DOI] [PubMed] [Google Scholar]
- Wieslander A., Rilfors L., Lindblom G. Metabolic changes of membrane lipid composition in Acholeplasma laidlawii by hydrocarbons, alcohols, and detergents: arguments for effects on lipid packing. Biochemistry. 1986 Nov 18;25(23):7511–7517. doi: 10.1021/bi00371a038. [DOI] [PubMed] [Google Scholar]
- Yamamoto A., Rouser G. Spectrophotometric determination of molar amounts of glycosphingolipids and ceramide by hydrolysis and reaction with trinitrobenzenesulfonic acid. Lipids. 1970 Apr;5(4):442–444. doi: 10.1007/BF02532112. [DOI] [PubMed] [Google Scholar]


