Abstract
Cerebral plaques containing β-amyloid (βA4) represent an invariant pathological feature of Alzheimer disease (AD). βA4 is proteolytically generated from its parent molecule, amyloid precursor protein (APP). In nonneuronal cells βA4 has been shown to be secreted via a pH-sensitive and endocytosis-dependent pathway, and this process, when occurring in the brain, is considered to play an important role in AD. In neurons the mechanisms of βA4 production are not known. Here we have analyzed these mechanisms by expressing human APP and its mutant versions in hippocampal neurons using the Semliki forest virus system. We show that these cells initially generate two pools of βA4, an extracellular and an intracellular, and only the extracellular pool is produced via a pH-sensitive and endocytosis-dependent pathway. Thus, hippocampal neurons are able to utilize an alternate pathway to produce intracellular βA4. We also show that a common feature of two types of APP mutations (“Swedish” and “London”) implicated in early-onset AD is their increased production of C-terminally elongated βA4 (β42), both intra- and extracellularly. Since neurons are the only cells that produce substantial levels of intracellular βA4 and also the main victims in AD, these findings may provide an important link between βA4 and neurodegeneration.
A causal role of the 4-kDa β-amyloid (βA4) peptide in the pathogenesis of Alzheimer disease (AD) is supported by several recent findings. Amyloid precursor protein (APP) mutations implicated in early-onset familial AD confer quantitative or qualitative changes in βA4 production (1–3) and transgenic mice harboring one of these mutants exhibit amyloid pathology in the brain (4). Down syndrome patients have an extra copy of the APP gene and develop early-onset AD. Amyloid pathology, however, precedes the onset of the disease by decades (5), indicating that it is a cause of the disease rather than a consequence. Two newly identified genes implicated in early-onset familial AD, presenilin 1 and 2, have been shown to increase βA4 production, especially the C-terminally elongated forms of βA4 both in vitro and in vivo (6). This C-terminally elongated βA4, which is especially prone to aggregation in vitro (7, 8), predominates in early stages of amyloid plaque formation in sporadic and familial AD as well as in Down syndrome cases (5, 9). These findings together with the invariant occurrence of amyloid plaques in AD brains suggest that all forms of AD share common pathways, the amyloid pathway being well supported by the existing data. Therefore, therapeutic interventions to this pathway are considered promising in the design of rational therapy or prevention of AD.
Several proteolytic cleavages have been shown to occur at the amyloid region of APP producing two major forms of 3- to 4-kDa fragments, namely βA4 and p3 (10). The N termini of βA4 and p3 are generated by β- and α-secretases, respectively, and the C termini by a γ-secretase (10) (see Fig. 1). These enzymes remain unidentified. APP is expressed at high levels in neurons, and these cells process APP in an amyloidogenic manner (11, 12) characterized by a high βA4/p3 ratio. Moreover, neurons are the only cells that have been shown to produce substantial amounts of cell lysate-associated βA4 (13). Because AD mainly affects neurons, hippocampus being one of the predilection sites, these cells should be a relevant model for studying APP metabolism. Here we have analyzed the βA4 production mechanisms of hippocampal neurons by expressing human wild-type APP (APPwt) and its mutant versions via the Semliki Forest virus (SFV) system.
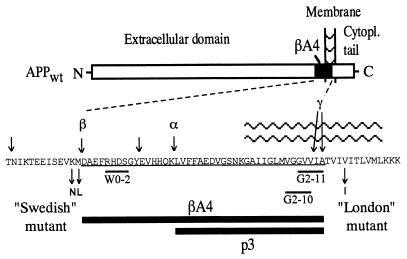
Figure 1.
Schematic presentation of cleavage sites on APP and two types of mutations implicated in early-onset familial AD. The amino acid sequence of βA4 (1–42) is underlined (one-letter code for amino acids). The major cleavage sites are indicated by arrows on the top of the sequence; the two mutations, βA4 and p3 fragments, and approximate location of the epitopes of W0-2, G2-10, and G2-11 antibodies are shown below the sequence.
MATERIALS AND METHODS
DNA Constructs and Preparation of SFV Particles.
Three mutations were introduced to APP695: a deletion of the cytoplasmic tail (APPΔCT), and two mutations implicated in early-onset familial AD, a “Swedish” and a “London” mutation (14, 15) (see Fig. 1). Mutagenesis was performed as described (16). SmaI digested wild-type and mutant APP695 cDNAs were cloned into the SmaI site of pSFV1 expression vector (17). pSFV1/APP and pSFV-helper DNAs were linearized with SpeI and in vitro transcribed (17) and cotransfected into baby hamster kidney cells using electroporation (18). The culture supernatant, containing infective recombinant SFV particles, was collected 36 h after the electroporation. The virus was concentrated and titrated as described (18).
Neuronal Culture and Infections.
Hippocampal neuron cultures were prepared from Wistar rat embryos as described (19). Briefly, dissociated neurons were plated on 3- or 6-cm plastic tissue culture dishes containing a monolayer of astrocytes and minimum essential medium supplemented with 10% fetal calf serum. After 4–8 h, the medium was changed into serum-free N2 medium (19). Proliferation of nonneuronal cells was prevented by 5 μM cytosine arabinoside (Calbiochem). The cultures were kept in a humidified incubator at 36.5°C/5% CO2 and cultured for 5–7 days before they were infected for 1 h with the recombinant SFV/APP (16). The SFV system permits rapid initiation of recombinant protein synthesis (within 2–3 h) after the 1-h infection period and does not produce significant cytopathic effects during the ≤7 h postinfection time course used here (18).
Metabolic Labeling Experiments.
Metabolic labeling of neurons cultured on 6-cm dishes was started at 2.5 h postinfection by changing the culture medium into 1.2 ml (or 2.4 ml in the medium-transfer experiments) of methionine-free minimum essential medium containing 300 μCi/ml (1 Ci = 37 GBq) [35S]methionine and 10% of N2 supplement. Conditioned medium (CM) and cells were harvested and immunoprecipitated as described (11). For the detection of βA4 and p3 a polyclonal antibody 692 (1:100 dilution) was used. This antibody precipitates equally βA4 and p3 and was raised against a synthetic peptide corresponding to residues 1–40 of βA4. For the detection of secretory APP (APPsec) and the C-terminal fragments of APP polyclonal antibodies 99294 and B12/4 were used, respectively (dilutions 1:1000). Antibody 99294 was raised against a bacterially produced APP fusion protein and B12/4 against a synthetic peptide corresponding to the last 20 amino acids of APP. βA4, p3, and the C-terminal fragments were electrophoresed in 12.5% Tris-tricine gels and APPsec in 6.5% Tris-glycine gels. After eletrophoresis the gels were processed as described (16) and quantified with the Fuji–Bas PhosphorImager system.
Immunoblotting and Quantification of Amyloid β40 and β42 Species.
Neurons cultured on 3-cm dishes were infected with SFV/APP and the cells (in 0.7 ml lysis buffer) and the CM (1 ml) were harvested at 6–7 h postinfection as described (11). For detection of total βA4, APPsec and holoAPP 200 μl of the CM and 150 μl of the cell lysates were immunoprecipitated with the mAb W0-2 (20) in the presence of protein G-agarose (Boehringer Mannheim). β40 and β42 species were detected by immunoprecipitation with the C terminus-specific mAbs G2-10 and G2-11 (20), respectively, in the presence of protein G-agarose. The epitopes of W0-2, G2-10, and G2-11 are shown in Fig. 1. The precipitation efficiency of 1–40 or 1–42 synthetic peptides by G2-10 and G2-11 was similar in the cell lysis buffer and in the N2 medium (data not shown). CM (200 μl) and cell lysate (150 μl) were used for precipitation with G2-10; double amounts were used for precipitation with G2-11. The precipitates were subsequently loaded on 10–20% Tris-tricine gels (Novex, San Diego) and the protein products were visualized from immunoblots with W0-2 antibody in conjunction with ECL detection system (Amersham). For quantification several exposures (from 30 sec to 50 min) of the films were analyzed with the Fuji–Bas PhosphorImager system. Quantifications were based on bands with unsaturated signal intensities.
RESULTS
Cell Lysate-Associated βA4 Is Mostly Intracellular.
In agreement with a previous study using a neuronal teratocarcinoma cell line (13), we were able to precipitate βA4 from the cell lysates of hippocampal neurons expressing human APPwt. Using a 4-h continuous labeling scheme cell lysate-associated βA4 constituted 13% (±3.5%) of the total βA4 produced by the cells. To test whether the cell lysate associated βA4 truly represents de novo generated intracellular βA4 we carried out medium-transfer experiments (Fig. 2A). We analyzed how much of the βA4 in the CM becomes associated with the cells via cell surface attachment or internalization. Hippocampal neurons expressing human APPwt were metabolically labeled for 3 h after which half of the medium was transferred to nonlabeled cells. Cell surface association or internalization of the protein was then analyzed 3–12 h later from the nonlabeled cell lysates by immunoprecipitation with the βA4 antibody 692. It was found that only trace amounts of βA4 could be recovered from the nonlabeled “recipient” cells compared with the “donor” cells (Fig. 2A). The βA4 signal obtained from the recipient cells was too low for accurate quantifications but remained below 20% of that found from the donor cells.
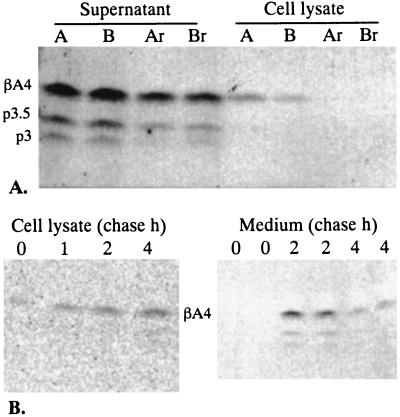
Figure 2.
Cell lysate-associated βA4 is mostly intracellular. (A) Recovery of βA4 from the CM of metabolically labeled cells to nonlabeled recipient cells. Neurons from dishes A and B were infected with SFV/APPwt and metabolically labeled in the presence of 300 μCi/ml [35S]methionine from 2.5 to 5.5 h postinfection. The CM of each dish was divided into paired aliquots (lanes A and Ar, B and Br, at 1.2 ml), one them was harvested (lanes A and B) along with the cell lysates, the other aliquots were transferred on intact recipient cells (lanes Ar and Br) supplemented with 500 μg/ml of cold methionine. The incubation on the recipient cells was continued for 3 (Ar) and 12 h (Br), after which the medium and the recipient cells were harvested. (B) Pulse-chase of βA4 harvested from the cell lysates of hippocampal neurons expressing SFV/APPwt. Cells were labeled for 30 min in the presence of [35S]methionine. The cell lysates were harvested at 0, 1, 2, and 4 h after the pulse. The medium was chased at 0–2 h and 2–4 h (fresh medium was added at 2 h).
Pulse-chase experiments were carried out to determine the kinetics of βA4 production (Fig. 2B). Immediately after a 30-min pulse (minimum time to allow reliable detection of βA4) we found trace amounts of βA4 in the cell lysates while it was undetectable in the medium. The secretion kinetics of βA4 and p3 followed that previously reported for APPsec in this system (12) with a peak during the first 2 h (0–2 h) of chase and decline thereafter (2–4 h). The cell lysate-associated βA4 showed gradual accumulation during the chase period from 0 to 4 h.
The above experiments demonstrate that very small amounts of βA4 of the medium become associated with the cells and that βA4 is detected in the cells before it appears in the medium. These data support the conclusion that the cell lysate-associated βA4 largely represents an intracellular de novo generated pool of molecules.
Distinct Pathways for Intracellular and Secreted βA4.
Previous studies in other cell types have demonstrated that secreted βA4, but not p3, is produced by a pH-sensitive and endocytosis-dependent mechanism (10, 21, 22). Whether this mechanism operates also in neurons is not known. Therefore, we tested the effect of NH4Cl, an alkalizing agent widely used in previous studies, on the production of the 3- to 4-kDa peptides in hippocampal neurons. In agreement with studies in other cells (21), the secretion of βA4 was down-regulated by NH4Cl, while there was no inhibition of p3 secretion (Fig. 3A). The secretion of the intermediate p3.5 fragment was also down-regulated by NH4Cl (Fig. 3A). Importantly, the inhibitory effect of NH4Cl on βA4 production was selective to the secretory βA4, there was no such down-regulation of the intracellular βA4 species (Fig. 3A). By analyzing the cytoplasmic tail containing C-terminal fragments (produced by APP secretases shown in Fig. 1), we found, upon NH4Cl treatment, a relative decrease in the 11- to 15-kDa fragments, the N termini of which extend beyond the α-cleavage site (Fig. 3B). We have previously radiosequenced these C-terminal fragments (11) and their N termini were shown to start at positions −12 (δ), +1 (β), and +11 (p3.5ct) according to βA4 numbering. The analysis of the C-terminal fragments illustrates that NH4Cl down-regulates the potential precursors of βA4 and p3.5.
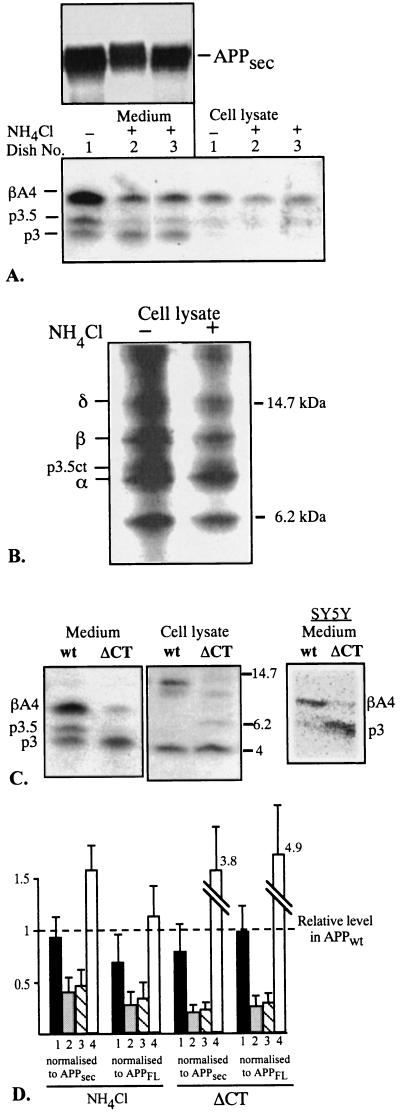
Figure 3.
pH- and endocytosis-dependence of βA4 production in hippocampal neurons expressing human APPwt. (A) Effect of NH4Cl on the production of secreted and intracellular 3- to 4-kDa peptides. Cells were metabolically labeled for 4 h in the presence or absence of NH4Cl (10 mM) after which the medium and cells were harvested and immunoprecipitated with the antibody 692. APPsec, precipitated with the 99294 antibody, is shown on the top. (B) Effect of NH4Cl on the C-terminal fragments of APP. The cleavage sites (δ, β, p3.5ct, and α) have been previously determined by radiosequencing (11). Cells were metabolically labeled for 4 h and immunoprecipitated with the B12/4 antibody. (C) Secreted and intracellular 3- to 4-kDa fragments from APPwt and the endocytosis deficient cytoplasmic tail deletion mutant APPΔCT. Cells were metabolically labeled for 4 h, and the medium and cell lysates were immunoprecipitated with the 692 antibody. Intracellular βA4 was detected by Western blot analysis with the W0-2 antibody (to avoid the signal from the α-cleaved 4-kDa fragment of the ΔCT mutant (p3 plus transmembrane domain). On the right is a PhosphorImager picture illustrating the secretion of βA4 and p3 in SY5Y cells expressing APPwt or APPΔCT. Stably transfected SY5Y cells were generated with the use of the pCEP4 expression vector (Invitrogen). These cells were metabolically labeled for 12 h; immunoprecipitation was with the 692 antibody. (D) Effect of NH4Cl treatment (n = 4) and expression of APPΔCT (n = 3) on the secreted 3- to 4-kDa peptides and intracellular βA4 after normalizing to the levels of APPsec and full-length APP (APPFL). The relative amount of each peptide in APPwt (n = 7) is fixed to 1 (shown by the dashed line). Columns: 1, intracellular βA4; 2, secreted βA4; 3, secreted p3.5; 4, secreted p3.
Endocytosis dependence of βA4 production was tested by expressing a mutant (APPΔCT) lacking 44 most C-terminal residues of the cytoplasmic tail of APP and thereby the endocytosis signals. Similar tail-less mutant has been shown to exhibit several-fold decreased endocytosis from the surface of CHO cells (23). Expression of this mutant in hippocampal neurons resulted in a marked down-regulation of both βA4 and p3.5 in the medium, while there was an up-regulation of p3 as compared with APPwt (Fig. 3C Left). However, no clear difference was observed in the levels of intracellular βA4 in APPΔCT as compared with APPwt-expressing cells (Fig. 3C Center). We also tested the effect of APPΔCT on βA4 secretion in human neuroblastoma SY5Y cells that were stably transfected with APPwt or APPΔCT. Similar down-regulation of βA4 was found in these cells upon expression of APPΔCT (Fig. 3C Right). No intracellular βA4 was detected in the SY5Y cells in these experiments (data not shown).
NH4Cl decreased APP secretion by ≈40% while expression of the APPΔCT mutant resulted in a ≈30% increased secretion of APP as compared with APPwt (data not shown, judged by APPsec/full-length APP ratio). Quantitative analysis of the 3- to 4-kDa peptides, after adjustment to the APPsec or full-length APP levels, illustrates that both NH4Cl treatment and the APPΔCT mutant resulted in down-regulation of secreted βA4 and p3.5 (Fig. 3D). In both cases there was no such effect on intracellular βA4 production (Fig. 3D). These results indicate that in hippocampal neurons the intracellular and secreted βA4 species are generated by distinct mechanisms. The secreted species is clearly NH4Cl-sensitive and endocytosis-dependent as reported in other cells (21, 22), while the intracellular species is not. Additionally, these data demonstrate that p3.5, an abundant product of hippocampal neurons, is also secreted via an NH4Cl-sensitive and endocytosis-dependent pathway.
Effect of APPSwe and APPLond on the Secreted and Intracellular Pools of βA4.
We analyzed the βA4 production of two APP mutations implicated in early-onset familial AD (14, 15). These mutations, termed “Swedish” (APPSwe) and “London” (APPLond), are illustrated in Fig. 1. It has been previously demonstrated that APPSwe favors β-cleavage at the expense of α-cleavage thus producing more secreted βA4 than APPwt (1, 2) whereas APPLond increases the amounts of C-terminally elongated βA4 species (β42/43) (3). We expressed APPwt, APPSwe, and APPLond in hippocampal neurons and measured total βA4 as well as the ratios of β40 and β42 in the secreted and intracellular pools. Total βA4, APPsec, and holoAPP were precipitated with a mAb W0-2, the proteins were subsequently detected on Western blots with W0-2 (Fig. 4A). The W0-2 antibody recognizes an epitope between residues 5 and 8 of βA4 (Fig. 1) and does not react with rodent APP or βA4 (20). The β40 and β42 species, which constitute the two major C-terminal variants of βA4, were analyzed by immunoprecipitating the samples with C terminus-specific mAbs G2-10 and G2-11, respectively (ref. 20; epitopes shown in Fig. 1). The proteins were detected with the W0-2 antibody on Western blots (Fig. 4A, lanes 2 and 3, respectively).
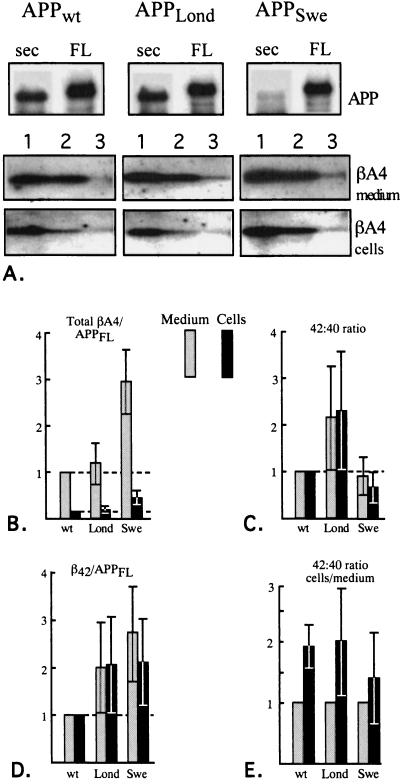
Figure 4.
Effect of APP clinical mutants on the production of βA4 and its C-terminal variants β40 and β42. (A) Detection of APPsec, holoAPP, total βA4, β40, and β42 in hippocampal neurons expressing APPwt, APPLond, and APPSwe. APPsec and APPFL are shown on the top, and the lanes numbered as 1 represent total βA4. The lanes numbered 2 represent β40, and those numbered 3 represent β42. (B) Total βA4 in the medium (filled) and cell lysates (hatched) after normalizing to holoAPP levels in neurons expressing APPwt, APPLond, and APPSwe (n = 8 each). Quantification was based on pair-wise comparisons between APPwt and the clinical mutants within each experiment. The levels of secreted and intracellular βA4 from APPwt are fixed to 1 and 0.15, respectively, which reflects the mean ratio between these two pools of βA4. (C) Comparison of 42/40 ratios between APPwt and the clinical mutants. The 42/40 ratio in the medium and cell lysates were analyzed separately; both are fixed at 1 in APPwt which is used as a reference (shown by the dashed line). APPLond vs. APPwt, P < 0.02 (medium) and P < 0.02 (cells); APPSwe vs. APPwt, P = not significant (medium) and P < 0.05 (cells); APPLond vs. APPSwe, P < 0.05 (medium) and P < 0.002 (cells). Student’s t test was used for pair-wise comparisons within each set of experiments (n = 9). APPLond vs. APPwt 1-tailed P values, other comparisons 2-tailed. (D) β42 levels in APPwt and the clinical mutants after normalizing to holoAPP. Medium and cell lysates were analyzed separately, and both are fixed to 1 in APPwt. APPLond vs. APPwt, P < 0.02 (medium) and P < 0.02 (cells); APPSwe vs. APPwt, P < 0.001 (medium) and P < 0.01 (cells); APPLond vs. APPSwe, P = not significant. Student’s t test for pair-wise comparisons (n = 8 each). (E) The 42/40 ratios in cells vs. medium of neurons expressing APPwt, APPLond, and APPSwe. The 42/40 ratio in the medium of each dish is fixed to 1. Cells vs. medium difference was analyzed by Student’s t test for pair-wise comparisons (n = 9 each): APPwt, P < 0.001; APPLond, P < 0.02; APPSwe, P = not significant (2-tailed P values).
Expression of APPSwe resulted in an increased secretion of total βA4 compared with APPwt as has been previously shown in neuronal (12) and nonneuronal (1, 2) cells, while APPLond had no effect on the secretion of total βA4 (Fig. 4B). The level of intracellular βA4 followed that of the secreted species (Fig. 4B). APPSwe exhibited a dramatic decrease in the amount of APPsec detectable with W0-2 antibody, indicating that very little α-cleaved APPsec was produced (Fig. 4A).
The ratios of β42 and β40 in the medium and cell lysates were compared in APPwt, APPLond, and APPSwe. The mean 42/40 ratio of APPwt was 0.11 in the medium and 0.19 in the cell lysates after correcting for the larger sample volume used for detection of β42. Because the efficiency of immunoprecipitation may be different with the two antibodies recognizing β40 and β42 these values should not be regarded as the exact ratios. Here we focus on the differences in 42/40 ratios in APPwt-, APPLond-, and APPSwe-expressing cells. APPLond produced a more than 2-fold mean increase in the 42/40 ratio in the medium (P < 0.02) as well as in the cell lysates (P < 0.02) when compared with APPwt (Fig. 4C). APPSwe exhibited a modest decrease (P = not significant) in 42/40 ratio in the medium as compared with APPwt, while a more pronounced, 33% decrease (P < 0.05) was observed in the cell lysates (Fig. 4C). Next we analyzed the net production of β42 in APPwt-, APPLond-, and APPSwe-expressing cells. In APPLond there was a 2-fold increase in β42 both in the medium and cell lysates; a similar or slightly higher increase of β42 was found in APPSwe (Fig. 4D). This illustrates that the overall increased production of βA4 in APPSwe also translates into increased β42 levels. Finally, we tested whether the 42/40 ratios differ in the intracellular vs. extracellular compartments. The 42/40 ratio of the cell lysate vs. medium of each dish, expressing APPwt, APPLond, and APPSwe, was compared. There was a 1.9- to 2.0-fold mean increase in the 42/40 ratio in the cell lysates compared with the medium upon expression of APPwt and APPLond (Fig. 4E) demonstrating a significantly increased recovery of β42 from the cell lysates. This effect was, however, not significant upon expression of APPSwe (Fig. 4E).
These results demonstrate that in the secreted and intracellular pools of βA4 APPLond exhibits a selective increase of β42 while in APPSwe there is an increase of both β40 and β42. Furthermore, neurons expressing APPwt and APPLond harbored a significantly higher fraction of β42 to the cell lysates compared with the CM.
DISCUSSION
We have shown here that cultured hippocampal neurons produce cell lysate-associated βA4 which mostly represent a de novo-generated intracellular pool of molecules. We demonstrated that the intracellular and secreted βA4 species derive by distinct pathways as judged by their different NH4Cl-sensitivity and endocytosis-dependence. The secreted pool of βA4 was generated via an NH4Cl-sensitive and endocytosis-dependent pathway, as has been shown in nonneuronal cells, while the intracellular pool was produced by an alternate pathway. The analysis of clinical APP mutants revealed that a common feature of APPLond and APPSwe is their increased generation of β42, both intra- and extracellularly. That two different APP mutations exhibit this same effect strengthens the hypothesis that β42 would play a central role in AD. Furthermore, the results with APPwt and APPLond indicate that β42 might constitute a higher fraction intracellularly than extracellularly. Since neurons are the main victims in AD, produce high levels of βA4, and represent the only cell type with abundant intracellular βA4, these results have important implications to AD.
We have used a viral expression system to express human APP in primary cultures of hippocampal neurons. A common problem with primary neuronal cultures is the recovery of insufficient amount of protein for biochemical analysis. Because these cells are resistant to transfection with other conventional methods we utilized the SFV system. This allowed us to produce sufficient amounts of APP for biochemical analyses and also to express various mutant forms of APP in these cells. SFV induces cytopathic effect after prolonged (>10 h) postinfection times that is a limitation of this system (18). However, the experiments we have carried out were designed so that the postinfection times did not exceed 7 h.
In other cell systems the pathway for βA4 secretion has been shown to depend on endocytosis of APP and acidic vesicular compartment (10, 21, 22). In nonneuronal cells it has been very difficult to detect intracellular βA4; it has been speculated that βA4 would be initially generated in endosomes but would be immediately secreted upon recycling of the vesicles back to the cell surface (10). Other routes for βA4 generation have been unraveled recently for APPSwe. This particular mutant is less dependent on endocytosis than APPwt (24), and at least the β-secretase cleavage of APPSwe can occur already in the constitutive secretory pathway (25, 26). Furthermore, upon overexpression of APPSwe it has been possible to detect intracellular βA4 in COS cells (27). In CHO cells it has been found that both secretory and endocytotic pathways contribute to the increased generation of βA4 from APPSwe (28). The cellular source of βA4 in AD plaques has been unclear. Recent studies have demonstrated that neurons or neuronal cells produce much higher βA4 levels than nonneuronal cells (11, 12). This suggests that neurons would be an important source of βA4 in AD brains. However, the mechanisms for βA4 generation have not been studied previously in neurons. Our finding that secreted βA4 follows a similar pH-sensitive and endocytosis-dependent pathway as has been found in other cells validates the use of nonneuronal cells in the characterization of this pathway. That there is a distinct pathway for the generation of intracellular βA4 is of major conceptual importance. These dual βA4 pathways in neurons may contribute differently to AD and might not be similarly inhibited by pharmacological strategies.
Our analysis of the clinical APP mutants demonstrated that in APPLond there is no increase of total βA4 as compared with APPwt, but the 42/40 ratio and thereby β42 is increased both in the secreted and intracellular pools. Thus, neurons exhibit a similar effect on β42 secretion as has been reported in other cells (3), and this effect is also found in the intracellular pool of βA4. APPSwe on the other hand increased both β40 and β42 fractions, while the 42/40 ratio was slightly decreased as compared with APPwt. Thus, the common feature of these two mutations is that they both induce a net increase of β42 in the intra- and extracellular compartments. These mutants would generate 2.0- and 2.7-fold more of β42 in the medium, and 2.0- and 2.1-fold more in the cell lysates, respectively, as compared with APPwt. Hence, the β42 load, generated by these mutants is close to each other. Interestingly, the mean age-of-onset of carriers of these mutations is also close to each other: APPLond (57 years) and APPSwe (53 years) (17, 18).
It is clear that the intracellular and extracellular pools of βA4 are not completely separate since the intracellular βA4 is known to become eventually secreted (29, 30). The intracellular fraction of 3- to 4-kDa peptides constituted almost entirely of βA4, only trace amounts of p3.5 but no p3 was detectable, even after longer exposure times. We observed a slightly increased recovery of β42 in relation to β40 from the cell lysates compared with the medium in APPwt- and APPLond-expressing neurons suggesting that β42 would constitute a larger fraction in the intracellular than in the secreted pool. This could enhance the pathogenic effects of intracellular βA4. An alternative explanation to the increased recovery of β42 from the cell lysates could be its increased cell surface association or internalization. However, if this were the case, we would have expected to find the same in APPSwe-expressing cells, which had the highest absolute levels of β42, but this was not observed. Using a human neuronal teratocarcinoma cell line elevated 42/40 ratios have also been found in the cells vs. medium (30). There are several possible explanations to these findings, including (i) enhanced production of β42 via the pathway for intracellular βA4, (ii) less efficient secretion of β42 compared with β40, (iii) differential stability of these peptides in the intra- and extracellular compartments, or (iv) conversion of β42 into β40 extracellularly. Further studies will be needed to determine the mechanism.
The existence of dual pathways for the generation of intra- and extracellular βA4 in neurons raises the question, which of these pathways is the one contributing to AD. A role of intracellular βA4 in AD has been, for technical reasons, difficult to address. Some insights into the potential role of intracellular βA4 have been gained from studies in mice with a transgenic βA4 (1–42) minigene (31). These mice exhibit neurodegeneration with several apoptotic features. This is of interest since several groups have reported apoptotic features also in AD brains (32, 33) and in cultures of Down syndrome neurons (34). A link between apoptosis and AD is also supported by the finding that a partial cDNA of the mouse homolog of the early-onset AD gene, presenilin-2, functions as an apoptosis inhibitor (35). Furthermore, enhanced rate of apoptosis has been reported in cells transfected with APP695 Val-642 mutations (such as APPLond) compared with APPwt-transfected cells (36). This effect could not be reproduced in APPwt-transfected cells by addition of 1–42 synthetic peptide or CM of the mutant-expressing cells (36). This suggests that extracellular βA4 does not explain this finding and provides a challenge to the amyloid hypothesis. However, the Val-642 mutant clearly produces a higher fraction of β42 than APPwt both in the secreted and intracellular pools. Hence, it is possible that this peptide actually exerts its effects intracellularly. A role of intracellular amyloid has been raised in context of another type of amyloidosis, the pancreatic amylin deposition of type 2 diabetes mellitus. Intracellular amylin deposition has been found to precede extracellular deposition in human insulinomas (37), and overexpression of amylin leads to intracellular amyloid formation and cytotoxicity in COS cells (38). Furthermore, cytotoxic effect of amylin requires conformational changes as has also been found with βA4 (12), and the affected cells exhibit apoptotic features (39). Taken together these findings indicate that intracellular βA4 can be considered as a potentially important contributor to AD pathogenesis, and as it is generated via an alternate pathway in hippocampal neurons, more work should be directed into the neuronal mechanisms of βA4 production.
Acknowledgments
We thank Liane Meyn for preparing the hippocampal cultures, Dr. Bart De Strooper for providing the B12/4 antiserum, and Dr. Tobias Hartmann and Joachim Stumm for discussion. P.J.T. was supported by a European Molecular Biology Organization postdoctoral fellowship, E.I. by a European Community Human Capital and Mobility fellowship, K.B. by a Bundesministerium für Bildung, Wissenschaft, Forschung and Technologie grant, and K.B. and C.G.D. by Deutsche Forschungsgemeinschaft (SFB 317) grants.
ABBREVIATIONS
- APP
amyloid precursor protein
- AD
Alzheimer’s disease
- SVF
Semliki Forest virus
- βA4
β-amyloid, APPsec, secretory APP
- APPwt
wild-type APP
- APPSwe
Swedish mutant of APP
- APPLond
London mutant of APP
- CM
conditioned medium
References
- 1.Citron M, Oltersdorf T, Haass C, McConlogue L, Hung A Y, Seubert P, Vigo-Pelfrey C, Liebergburg I, Selkoe D J. Nature (London) 1992;360:672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- 2.Cai X D, Golde T E, Younkin S G. Science. 1993;259:514–516. doi: 10.1126/science.8424174. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki N, Cheung T T, Cai X-D, Odaka A, Otvos L, Jr, Eckman C, Golde T E, Younkin S. Science. 1994;264:1336–1340. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- 4.Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, et al. Nature (London) 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 5.Teller J K, Russo C, Debusk D M, Angelini G, Zaccheo D, Dagnabricarelli, Scartezzini P, Bertolini S, Mann D M A, Tabaton M, Gambetti P. Nat Med. 1996;2:93–95. doi: 10.1038/nm0196-93. [DOI] [PubMed] [Google Scholar]
- 6.Scheuner D, Eckman C, Jensen M, Song X, Citron M, et al. Nat Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 7.Hilbich C, Kisters-Woike B, Reed J, Masters C L, Beyreuther K. J Mol Biol. 1991;218:149–163. doi: 10.1016/0022-2836(91)90881-6. [DOI] [PubMed] [Google Scholar]
- 8.Jarrett J T, Berger E P, Landsbury P T. Biochemistry. 1993;32:4693–4697. doi: 10.1021/bi00069a001. [DOI] [PubMed] [Google Scholar]
- 9.Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y. Neuron. 1994;13:45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- 10.Selkoe D J. Curr Opin Neurobiol. 1994;4:708–716. doi: 10.1016/0959-4388(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 11.Simons M, De Strooper B, Multhaup G, Tienari P J, Dotti C G, Beyreuther K. J Neurosci. 1996;16:899–908. doi: 10.1523/JNEUROSCI.16-03-00899.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Strooper B, Simons M, Mulhaup G, Van Leuven F, Beyreuther K, Dotti C G. EMBO J. 1995;14:4932–4938. doi: 10.1002/j.1460-2075.1995.tb00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wertkin A M, Turner R S, Pleasure S J, Golde T E, Younkin S G, Trojanowski J Q, Lee V M. Proc Natl Acad Sci USA. 1993;90:9513–9517. doi: 10.1073/pnas.90.20.9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mullan M, Crawford F, Axelman K, Houlden H, Lilius L, Winblad B, Lannfelt L. Nat Genet. 1992;1:345–347. doi: 10.1038/ng0892-345. [DOI] [PubMed] [Google Scholar]
- 15.Goate A, Chartier-Harlin M-C, Mullan M, Brown J, Crawford F, et al. Nature (London) 1991;349:704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- 16.Tienari P J, De Strooper B, Ikonen E, Simons M, Weidemann A, Czech C, Hartmann T, Multhaup G, Masters C L, Van Leuven F, Beyreuther K, Dotti C G. EMBO J. 1996;15:5218–5229. [PMC free article] [PubMed] [Google Scholar]
- 17.Liljeström P, Garoff H. BioTechnology. 1991;9:1356–1361. doi: 10.1038/nbt1291-1356. [DOI] [PubMed] [Google Scholar]
- 18.Olkkonen V M, Liljeström P, Garoff H, Simons K, Dotti C G. J Neurosci Res. 1993;35:445–451. doi: 10.1002/jnr.490350412. [DOI] [PubMed] [Google Scholar]
- 19.Goslin K, Banker G A. In: Culturing Nerve Cells. Banker G A, Goslin K, editors. Cambridge, MA: MIT Press; 1991. pp. 251–281. [Google Scholar]
- 20.Ida N, Hartmann T, Pantel J, Schröder J, Zerfass R, Förstl H, Sandbrink R, Masters C L, Beyreuther K. J Biol Chem. 1996;271:22908–22914. doi: 10.1074/jbc.271.37.22908. [DOI] [PubMed] [Google Scholar]
- 21.Haass C, Hung A Y, Schlossmacher M G, Teplow D B, Selkoe D J. J Biol Chem. 1993;268:3021–3024. [PubMed] [Google Scholar]
- 22.Koo E H, Squazzo S L. J Biol Chem. 1994;269:17386–17389. [PubMed] [Google Scholar]
- 23.Koo E H, Squazzo S L, Selkoe D J, Koo C H. J Cell Sci. 1996;109:991–998. doi: 10.1242/jcs.109.5.991. [DOI] [PubMed] [Google Scholar]
- 24.Citron M, Teplow D B, Selkoe D J. Neuron. 1995;14:661–670. doi: 10.1016/0896-6273(95)90323-2. [DOI] [PubMed] [Google Scholar]
- 25.Haass C, Lemere C A, Capell A, Citron M, Seubert P, Schenk D, Lannfelt L, Selkoe D J. Nat Med. 1995;1:1291–1296. doi: 10.1038/nm1295-1291. [DOI] [PubMed] [Google Scholar]
- 26.Thinakaran G, Teplow D, Siman R, Greenberg B, Sisodia S S. J Biol Chem. 1996;271:9390–9397. doi: 10.1074/jbc.271.16.9390. [DOI] [PubMed] [Google Scholar]
- 27.Perez R G, Squazzo S L, Koo E. J Biol Chem. 1996;271:9100–9107. doi: 10.1074/jbc.271.15.9100. [DOI] [PubMed] [Google Scholar]
- 28.Martin B L, Schraderfischer G, Busciglio J, Duke M, Paganetti P, Yankner B A. J Biol Chem. 1995;270:26727–26730. doi: 10.1074/jbc.270.45.26727. [DOI] [PubMed] [Google Scholar]
- 29.Fuller S J, Storey E, Li Q X, Smith A I, Beyreuther K, Masters C L. Biochemistry. 1995;34:8091–8098. doi: 10.1021/bi00025a015. [DOI] [PubMed] [Google Scholar]
- 30.Turner R S, Suzuki N, Chyung A S C, Younkin S G, Lee V M-Y. J Biol Chem. 1996;271:8966–8970. doi: 10.1074/jbc.271.15.8966. [DOI] [PubMed] [Google Scholar]
- 31.LaFerla F M, Tinkle B T, Biedrich C J, Haudenschild C C, Jay G. Nat Genet. 1995;9:21–30. doi: 10.1038/ng0195-21. [DOI] [PubMed] [Google Scholar]
- 32.Lassmann H, Bancher C, Breitschopf H, Wegiel J, Bobinski M, Jellinger K, Wisniewski H M. Acta Neuropathol. 1995;89:35–41. doi: 10.1007/BF00294257. [DOI] [PubMed] [Google Scholar]
- 33.Andersson A J, Su J H, Cotman C W. J Neurosci. 1996;15:1710–1719. doi: 10.1523/JNEUROSCI.16-05-01710.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Busciglio J, Yankner B A. Nature (London) 1995;378:776–779. doi: 10.1038/378776a0. [DOI] [PubMed] [Google Scholar]
- 35.Vito P, Lacana E, D’Adamio L. Science. 1996;271:521–525. doi: 10.1126/science.271.5248.521. [DOI] [PubMed] [Google Scholar]
- 36.Yamatsuji T, Okamoto T, Takeda S, Murayama Y, Tanaka N, Nishimoto I. EMBO J. 1996;15:498–509. [PMC free article] [PubMed] [Google Scholar]
- 37.O’Brien P C, Butler A E, Johson K, Butler P C. Diabetes. 1994;43:329–336. doi: 10.2337/diab.43.2.329. [DOI] [PubMed] [Google Scholar]
- 38.O’Brien T, Butler P C, Kreutter D K, Kane L A, Eberhardt L. Am J Pathol. 1995;147:609–616. [PMC free article] [PubMed] [Google Scholar]
- 39.Lorenzo A, Razzaboni R, Weir G C, Yankner B A. Nature (London) 1994;368:756–760. doi: 10.1038/368756a0. [DOI] [PubMed] [Google Scholar]