Abstract
A preparation of decapitated Drosophila melanogaster has been used for direct application of drugs to the nerve cord. Serotonin, dopamine, and octopamine stimulate locomotion and grooming, showing distinguishable effects that often are potentiated by addition of the vertebrate monoamine oxidase-inhibitor hydrazaline. Many of the hydrazaline-induced effects are sexually dimorphic, with males showing greater responses than females. Behaviors similar to those induced by dopamine can be induced by application of the vertebrate dopamine D2-like receptor agonist quinpirole, whose effects are also sexually dimorphic. In contrast, vertebrate D2-like and D1-like dopamine antagonists result in akinesic states, and D1-like agonists selectively stimulate grooming. These data indicate that Drosophila nerve cord amine receptors are coupled to reflexive behaviors similar to those stimulated by brain dopamine receptors in vertebrates.
Keywords: stereotypies, receptors, grooming, locomotion
In both vertebrates and invertebrates, the basic neural oscillators controlling reflex behaviors and locomotion are contained within the spinal cord, or the nerve cord in the case of invertebrates (reviewed in refs. 1–6). Several nonhuman vertebrates show both locomotion and reflex scratching behaviors in response to irritants even after the spinal cord is cut. Similarly, decapitated insects show a basal level of spontaneous grooming as well as a normal grooming response when a sensory bristle is stimulated by gentle mechanical contact (5, 6). Studies in both Drosophila and larger insects show that the grooming response consists of a stereotyped series of leg, wing, and body movements that result in removal of debris from the legs and body of the fly (6–8).
Many of these behaviors can be stimulated by application of biogenic amines. Injection of l-DOPA and 5-HTP, the precursors for dopamine/noradrenaline and serotonin, respectively, can initiate walking motor patterns in spinal cats (9–11) and rabbits (12), although a more recent study shows that adrenergic agents are the most effective (13). Similarly, in partly dissected preparations of moth and locust, addition of dopamine or octopamine to the thoracic ganglia leads to stimulation of flight motor and stepping oscillators (14–16), and in cockroaches, dopamine can stimulate an escape response (17). These applications of exogenous biogenic amines likely mimic the roles of amines and/or noradrenaline provided to the nerve or spinal cord from the brain by descending projections that are found in both vertebrates and insects (18, 19). The invertebrate nerve cord differs from the higher vertebrate spinal cord in that the nerve cord contains aminergic cells bodies (20–23). Presumably these cell bodies provide localized sources of amines to regions of the nerve cord neuropil that are not accessed by the descending aminergic projections.
In vertebrates and invertebrates, dopamine and other amines act through families of G protein-linked seven transmembrane receptors (reviewed in refs. 24–26). Stimulation or inhibition of vertebrate dopamine receptors in the brain by class-specific agonists can either stimulate or repress locomotion and behaviors known as stereotypies, reflexive and abnormally repetitive behaviors such as grooming, sniffing, rearing, and chewing. The dopamine receptors can be classified into two general classes of receptors, the D1-like and D2-like receptors, which differ in structure, in how they are linked to adenyl cyclase, and in the physiological responses that they mediate (for a review see ref. 24).
A severe problem limiting behavioral studies of drugs affecting amine function in insects is that the insect central nervous system is covered by a neuroepithelium, such that injection of drugs is often ineffective (ref. 27; unpublished results). Here we describe a behaviorally active preparation of decapitated Drosophila that allows direct addition of drugs to the nerve cord. This preparation, which has been used previously in studies of mating preference (28, 29), sensory innervation (5), and learning (30), can remain active for several days if kept moist. The decapitated flies maintain a normal standing posture and show a low level of spontaneous grooming as well as a provoked grooming response and a vigorous righting response. We have used this preparation to study the behavioral pharmacology of these responses. We find that dopamine, octopamine, and serotonin stimulate locomotion and grooming, and that drugs specific for vertebrate dopamine receptors can modulate these behaviors. Additionally, many of these agents show sexual dimorphisms in their responses. These observations open a new preparation for functional studies of amine receptors in a genetically tractable model system.
MATERIALS AND METHODS
Decapitation of Flies and Behavioral Assays.
Decapitated flies of w1118 Drosophila melanogaster were prepared by cutting heads from CO2-anesthetized flies with Dewecker Iris scissors (Fullam, Schenectady, NY). Flies used for decapitation were maintained on a 12:12 light/dark cycle, and studies were performed near the middle of their subjective day. Flies younger than 8 h posteclosion were not used, and the majority of flies used in these studies were ages 8–30 h. Exposure to CO2 during decapitation was minimized and limited to 5 min. The decapitated flies were allowed to recover for 0.5 to 2 h in a humidified container; with short exposure to CO2 anesthesia, responses are constant over this time period. Only those decapitated flies that showed an upright posture and an evoked grooming response after stimulation of a thoracic bristle with a single hair of a fine paintbrush were used in further studies.
Drugs were made up in 10 mM NaPi, pH 6, or in the case of SCH 23390, water, as recommended by the manufacturer. Drugs were applied to the exposed nerve cord at the anterior notum as a droplet with a micropipettor, maintaining contact for 4–5 sec. Room temperature was maintained at 22–24°C, and illumination was through a fiber optic illuminator equipped with a heat filter to maintain constant temperature on the viewing stage. Grooming by the hindlegs and forelegs was monitored separately, as were locomotion, responsiveness to mechanical stimulation, and hyperactivity. These behaviors were assayed over a 2-min observation interval, which initiated immediately after drug addition, as determined by monitoring videotapes of the treated flies on a grid of 1-mm graph paper. Locomotion was measured by counting grid crossings, assigning a value of 1.4 for diagonal crossings. The observer quantitating the behaviors was unaware of the drugs given. Grooming was counted only when the given legs were moving and in contact with either the legs or the body. For brevity we call grooming by the hindlegs “hindleg grooming.” Hyperactive behavior was counted as the time the animals lacked motor control, evidenced by high levels of leg activity and/or wing buzzing causing them to fall over or flip upside down. For all drugs used in this study, lower concentrations of drugs produced the same behaviors, but with lessened frequency and/or with an increased delay of onset. For each datapoint in this study, ≈15 flies of each sex were examined unless otherwise noted. For drugs showing significant sex dimorphisms, the data are shown separately as a function of sex; otherwise, the data are shown pooled.
Biogenic amines were used at 10 mM in 10 mM NaPi, pH 6, unless otherwise noted. Agonists and antagonists were used at the following concentrations unless otherwise noted: hydrazaline, 1 mM; SK 82958, 5 mM; SCH 23390, 2 mM; quinpirole, 5 mM; eticlopride, 5 mM.
All receptor agonists and antagonists were from Research Biochemicals, and amines were from Sigma. Activities of the drugs used in this study are summarized in Table 1.
Table 1.
Activities of drugs used in this study
| Drug | Vertebrate activity | Activity in decapitated flies |
|---|---|---|
| SCH 23390, SKF 85366 | D1-like antagonist | Akinesia, extended posture, tremor |
| SKF 82958, SKF 81297 | D1-like agonist | Hindleg grooming |
| Eticlopride, raclopride | D2-like antagonist | Akinesia, contracted posture |
| Quinpirole | D2-like agonist | Locomotion, hindleg grooming, hyperactivity |
| Hydrazaline | Monoamine–oxidase inhibitor | Potentiates amine responses |
Tremor frequency was estimated by examining leg position in sequential video frames.
Statistical Analyses.
One-, two-, or three-way type I sums of squares ANOVA analyses were performed using the general linear models procedure of SAS Institute (Cary, NC) (31). The datasets were subjected to a square root transformation to approximate normality (32). A sequential Bonferroni test was applied (33) to determine significance of the resulting probability values. All significant differences marked in the figures pass the sequential Bonferroni test, except for one instance described in Fig. 1, in which significance was shown using Fisher’s Exact Test (32). All figures shown in this manuscript present the means ± SEM of the untransformed datasets.
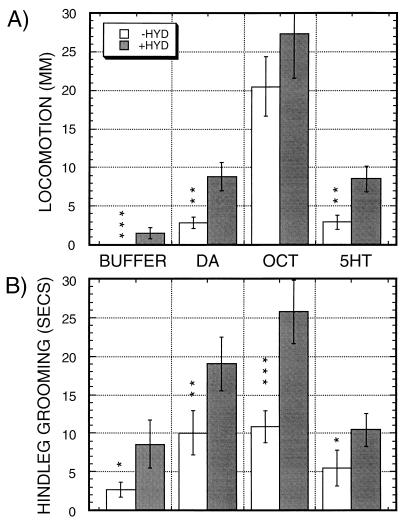
Figure 1.
Amines and hydrazaline stimulate locomotion (A) and hindleg grooming (B) in decapitated flies. Locomotion and hindleg grooming was assayed in a 2-min observation time after additions of amines (open bars) or amines in the presence of 1 mM hydrazaline (shaded bars). Solutions added were BUFFER: buffer control; DA: dopamine; OCT: octopamine; 5HT: serotonin. Data show the means ± SEM from n = 30 observations for each condition, except for n = 90 for those exposed to buffer or buffer plus hydrazaline. Significance levels for differences as a function of hydrazaline are indicated by asterisks, using square root transformed datasets for calculations (see Materials and Methods). ∗, P ≤ 0.05; ∗∗, P ≤ 0.005; ∗∗∗, P < 0.0005. Significance of the stimulation of locomotion by hydrazaline compared with buffer alone was determined by comparing the fractions of flies moving ≥1 mm. Nine of 29 flies locomote after hydrazaline exposure, versus none of 90 exposed to buffer alone (P < 0.0001, Fisher’s Exact Test).
RESULTS
To determine the effects of exogenous biogenic amines, we added amines to the cut nerve cord of decapitated flies. Locomotion and foreleg and hindleg grooming were assayed in flies for 2-min intervals after application of buffer or 10 mM amines either alone (Fig. 1, open bars), or in conjunction with 1 mM hydrazaline (Fig. 1, black bars), an inhibitor of vertebrate monoamine oxidase.
Addition of these compounds induces a set of complex behavioral responses in the decapitated flies, with significant stimulation of locomotion (Fig. 1A) and hindleg grooming (Fig. 1B) by the amines dopamine and octopamine, with octopamine showing a particularly strong stimulation of locomotion. Each of the locomotor responses is significant to P ≤ 0.007 when comparing amine vs. buffer. When only buffer was added, absolutely no locomotion was observed in the 90 decapitated flies examined. Serotonin has a milder effect than the other amines. Locomotion is significantly stimulated (P = 0.007), but the stimulation of hindleg grooming is not significant (P = 0.16). Stimulation of hindleg grooming by octopamine and dopamine are both significant to P ≤ 0.001. We also tested dopa, norepinephrine, and tyramine in this assay (data not shown). Dopa and norepinephrine showed no significant effects, and tyramine, the metabolic precursor to octopamine, generates behaviors similar to, but much milder than, the behaviors generated by octopamine, potentially explained by in vivo metabolism to octopamine. Only slight effects on grooming by the forelegs were observed (data not shown), and grooming by the middle legs was rarely observed and was not quantitated. Many of the drug-induced behaviors in the decapitated flies can be viewed as video clips at http://minerva.acc.virginia.edu/~biology/Fac/Hirsh.html (or on the tapes provided for the reviewers).
The hindleg grooming that is stimulated by each of the drugs consists of a subset of the normal hindleg grooming response. Grooming of the wings, abdomen, and hindlegs is frequently observed, but grooming of the midlegs is rarely observed. This latter grooming behavior is seen in intact flies (7). The locomotion that results from these additions looks qualitatively similar to normal locomotion, albeit slower than in intact flies. The locomotion is slow and deliberate, and the flies often will move at a constant turning angle; i.e., many flies will move in either straight lines or in circles, but usually will not vary their path unless they collide with an obstruction. There is an abnormal aspect to the interaction of locomotion and grooming. In intact flies, grooming and locomotion are mutually exclusive activities (7). In decapitated flies that show strong locomotor responses there is frequently concomitant locomotion with the forelegs and midlegs during bouts of hindleg grooming.
In addition to the quantitative differences shown above, there are additional differences in the responses that distinguish the responses to each amine. Octopamine leads to rapid wing flicks with ≈0.5-sec interval in ≈50% of the flies, often continuing for the entire observation period, a behavior that is not observed with either serotonin or dopamine. Additionally, dopamine-treated animals show significant amounts of hyperactive behavior, spending 11 ± 5 sec of each 2-min observation period in a hyperactive state, with 5 of 29 flies showing hyperactivity [P = 0.008; Fisher’s Exact Test (32)]. This behavior was not observed with either serotonin or octopamine in observations of 28 or 43 flies, respectively. From these results we conclude that the amines dopamine, octopamine, and serotonin are leading to distinguishable responses in the decapitated flies.
The major degradative route for dopamine and other biogenic amines in vertebrates is via monoamine oxidase enzymes. Only extremely low levels of these enzymes have been detected in Drosophila (34), and monoamine–oxidase activity is not detectable in many other insects (35). We added 1 mM hydrazaline, an inhibitor of vertebrate monoamine oxidases, in conjunction with addition of buffer or amines, to see whether this treatment would potentiate the responses (Fig. 1, black bars). Addition of hydrazaline with either buffer or amines leads to increases in both locomotion and hindleg grooming, with significant increases indicated by asterisks in Fig. 1. Hydrazaline stimulates hindleg grooming when added either alone or in conjunction with each of the amines (Fig. 1B), and significantly stimulates locomotion when added in conjunction with either dopamine or serotonin (Fig. 1A).
We suspect that hydrazaline acts by increasing levels of endogenous amines and by increasing the effective dose of exogenously applied amine. Consistent with this mode of hydrazaline action, injection of 10 mM hydrazaline into third instar larvae results in lethality with dark pigmentation (not shown), and treatment of the decapitated flies with ≥5 mM hydrazaline leads to increased locomotion alternating with periods of hyperactivity (data not shown). The effects we observe are complex given that hydrazaline may increase levels of all endogenous amines, but the potentiation of locomotion and hindleg grooming nonetheless is consistent with our postulated mode of hydrazaline action.
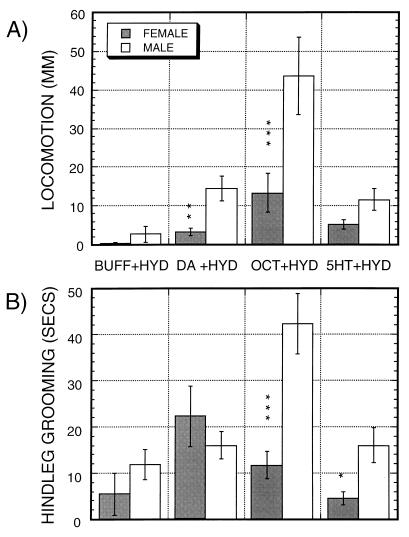
Many of the effects of hydrazaline on locomotion and hindleg grooming show sexual dimorphisms (Fig. 2), with significant differences marked by vertical asterisks in Fig. 2. For all of the significant differences, males show increased activity relative to females. The most striking dimorphisms in both locomotion and grooming are induced by octopamine in the presence of hydrazaline, which stimulates these behaviors 3- to 4-fold as strongly in males as in females. No significant sexual dimorphisms are observed when amines are added without hydrazaline (data not shown).
Figure 2.
Sexual dimorphisms in the action of hydrazaline (HYD) on decapitated flies when added either alone (BUFF) or in the presence of 10 mM dopamine (DA), octopamine (OCT), or serotonin (5HT). Effects on locomotion (A) and hindleg grooming (B). Data are from the same observations as in Fig. 1, except that effects are plotted separately for each sex. n = 15 observations for each mean.
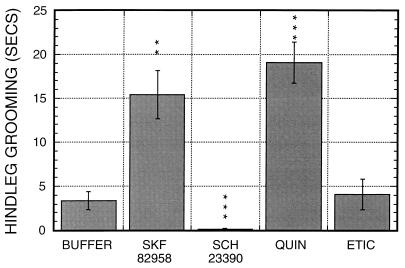
We next examined whether agents active on vertebrate amine receptors would be active in this system. A number of serotonin receptor agonists and antagonists showed only minor effects on the decapitated flies (data not shown), but a number of drugs affecting vertebrate dopamine receptors show striking effects. These effects are summarized in Table 1. A D1-like agonist, SKF 82958, leads to a ≈5-fold stimulation of hindleg grooming as compared with buffer (Fig. 3; P < 0.001), with absolutely no stimulation of locomotion (data not shown) or additional behaviors. Another D1-like agonist, SKF 81297, shows similar responses (data not shown). A D1-like antagonist, SCH 23390, produced a striking phenotype of akinesia associated with a 10- to 15-Hz tremor, with a total loss of responsiveness to mechanical stimulation. Within ≈10 sec of drug addition, spontaneous grooming movements cease, and the flies become unresponsive to mechanical provocation, losing the ability to right themselves. The treated flies often fall on their sides with distally extended wings and outstretched legs, a phenotype resembling heavy CO2 sedation, and do not recover from this state. An identical phenotype also was seen with the D1-like antagonist SKF 85366.
Figure 3.
Effects of vertebrate dopamine agonists/antagonists on hindleg grooming in decapitated flies. Flies were exposed to Buffer: NaPi, pH 6.0; SK 82958, a D1-like dopamine receptor agonist; SCH 23390, a D1-like dopamine receptor antagonist; quinpirole, a D2-like dopamine receptor agonist; and eticlopride, a D2-like dopamine receptor antagonist. n = 28 to 32 for each drug. Quinpirole, SCK 82958, and SCH 23390 each have highly significant effects on hindleg grooming. The low level of hindleg grooming remaining after SCH 23390 treatment resulted from one fly that groomed for 2 sec immediately after application of the drug. There is no significant sex specificity for any of the effects shown (data not shown).
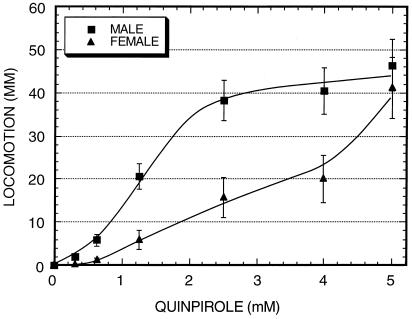
A D2-like agonist, quinpirole, shows strong effects on both hindleg grooming and locomotion. At 5 mM quinpirole, levels of hindleg grooming are stimulated ≈6-fold (Fig. 3), and locomotion is stimulated strongly (Fig. 4). The quinpirole-treated flies also show significant periods of hyperactive behaviors, similar to the hyperactivity induced by dopamine. When exposed to 5 mM of quinpirole males were hyperactive for 15 ± 10 secs, whereas females showed no hyperactivity. At higher quinpirole concentrations, hyperactivity is seen in both sexes (data not shown).
Figure 4.
Locomotion as a function of quinpirole concentration in decapitated males and females. Decapitated flies were exposed to quinpirole at the indicated concentrations, and locomotion was assayed in the subsequent 2-min interval. n = 13 to 15 for each datapoint.
A D2-like antagonist, eticlopride, shows no significant effects on hindleg grooming when added at 5 mM (Fig. 3), but leads to a loss of responsiveness. When eticlopride-treated flies were overturned, none of 20 flies were able to right themselves and lacked any of the vigorous righting behaviors that normally are observed. When flies were exposed to 10 mM of eticlopride, 18 of 35 flies showed an extreme phenotype in which they fell on their sides or upside down with legs withdrawn and almost motionless, and were totally unresponsive to mechanical provocation. There is no visible tremor as seen with D1-like antagonists. Similar results were observed with the D2-like antagonist raclopride.
The similarity in the responses to dopamine and quinpirole led us to examine the sex specificity of the quinpirole response. Fig. 4 shows the locomotor response for each sex to quinpirole as a function of quinpirole concentration. Maximal levels of locomotion of ≈45 mm per 2 min are attained in both sexes at 5 mM of quinpirole. At higher quinpirole concentrations hyperactive behaviors predominate and limit overall locomotion. At low quinpirole concentrations, males are selectively stimulated by quinpirole, with the concentration of quinpirole leading to half-maximal activity differing by 3- to 4-fold in males vs. females. This is most simply consistent with a sex-specific increase in receptor affinity in males vs. females, although more complicated explanations, such as expression of different receptor types in males vs. females, cannot be discerned until the relevant receptors are identified.
DISCUSSION
We show that decapitated flies are an active preparation for the study of neurally acting drugs. In this study we show that the biogenic amines dopamine, serotonin, and octopamine can stimulate grooming, locomotion, and hyperactive behaviors in similar, but distinct, manners. Actions of the amines, and particularly dopamine, can be mimicked by drugs that stimulate vertebrate dopamine receptors, and antagonists lead to akinesic states. Additionally, we see several striking sexual dimorphisms in the activities of the amines and quinpirole, with greater activity in males than in females.
What Receptors Mediate the Observed Behaviors?
We expect that the receptors mediating the observed responses in the decapitated flies are in the nerve cord rather than in the periphery, because we have been unsuccessful in generating strong responses by direct injection into the adult hemolymph of intact flies (unpublished data). None of the heretofore-identified amine receptors in Drosophila are good candidates for mediating the observed responses. Two D1-like dopamine receptors (36–39), one octopamine/tyramine receptor (40, 41), and a number of serotonin receptors (25) have been identified in Drosophila. D2-like dopamine receptors have not been isolated from Drosophila, but if highly divergent in sequence from vertebrate receptors, they may have eluded detection. Both of the D1-like dopamine receptors are localized primarily, if not exclusively, to the brain (36, 39), thus excluding them from consideration. The locust homolog of the Drosophila tyramine/octopamine receptor is expressed widely in the central nervous system, including in the nerve cord (42). However, the Drosophila tyramine/octopamine receptor responds more strongly to tyramine than octopamine for adenylyl cyclase inhibitory responses (40), and equally to both compounds when cytosolic Ca2+ release is assayed (43). These results are not consistent with the weak effects of tyramine relative to octopamine in the decapitated flies.
Our observations that vertebrate D1-like and D2-like dopamine receptor agonists stimulate distinct behaviors are most simply compatible with these agents interacting with distinct receptors. The vertebrate D1-like agonists SKF 82958 and SKF 81297 selectively stimulate hindleg grooming, whereas the D2-like agonist quinpirole stimulates both hindleg grooming and locomotion. Similarly, D1-like and D2-like antagonists lead to distinguishable akinesic and unresponsive states. Decapitated flies treated with D1-like antagonists show an akinesic state with an associated tremor and extended posture, whereas D2-like antagonists cause an akinesic state without tremor and with contracted legs.
Are the vertebrate dopamine receptor agonists and antagonists interacting with Drosophila dopamine receptors or with other types of amine receptors? Our best evidence on this issue comes from comparing behaviors stimulated by the dopamine receptor agonists with the behaviors stimulated by the amines. The fullest behavioral responses to dopamine agonists are seen with the D2-like agonist quinpirole. These responses bear overlapping resemblances to the responses to dopamine, serotonin, and octopamine, but with the best qualitative resemblance to the dopamine-stimulated behaviors. Quinpirole leads to high levels of locomotor activity and hindleg grooming with periods of hyperactivity. Similar behaviors are induced by dopamine, although the magnitude of responses is smaller. The response to octopamine is similar in that locomotion and grooming are strongly stimulated, but differs from quinpirole responses in that hyperactive behaviors are not observed, and many flies show rapid wing scissoring. Serotonin generates rather weak locomotor and grooming responses and also does not lead to hyperactivity.
Even given the above arguments, until the receptor(s) mediating these responses are identified we cannot eliminate the possibility that multiple amines could be interacting with a single novel receptor subtype and activating it in different manners. Evidence for such a novel receptor has been generated from binding studies using honeybee brain extracts (44). These workers identified a D2-like antagonist binding activity that could be competed more strongly by octopamine and tyramine than by dopamine.
Similarities in the Responses to Dopamine Agonists/Antagonists Between Drosophila and Vertebrates.
The responses of decapitated Drosophila show many resemblances to the effects of vertebrate dopamine receptor agonists and antagonists after injection into rodents. Both D1-like and D2-like receptor agonists stimulate locomotion and sterotyped reflexive behaviors, with differences in the types of stereotypies induced (45), and both D1-like and D2-like antagonists lead to an akinesic state (24, 46, 47). Stereotyped behaviors include behaviors such as grooming, sniffing, chewing, and posturing that are controlled by spinal cord neural oscillators. In our preparations of decapitated Drosophila, we see stimulation of both hindleg grooming and locomotion with the D2-like agonist quinpirole, whereas D1-like agonists selectively stimulate hindleg grooming without stimulating locomotion. D1-like antagonists lead to a totally akinesic state accompanied by tremor, and D2-like antagonists show a similar, but distinguishable, phenotype of loss of motility and responsiveness, but with no detectable tremor and a different body posture.
These phenotypic similarities are striking, indicating that if these compounds are interacting with receptors analogous to the vertebrate dopamine receptors, that the link to locomotor and stereotyped grooming behaviors occurred very early in evolution, before the split between Drosophila and vertebrates. We know of only one previous study showing results that may be analogous in an invertebrate. Treatment of planaria with D1-like or D2-like agonists results in distinguishable hyperkinetic or postural states that can be inhibited by class-specific antagonists (48).
One important and obvious distinction between the results obtained in vertebrates and in decapitated Drosophila is that the brain has been removed from the latter. Most vertebrate behavioral responses to dopamine depend on dopamine receptors in the brain. There are two explanations possible for the similarities in behavioral responses in these systems. First, it is possible that the insect nerve cord contains functions that have been taken over by the brain in higher vertebrates. Alternatively, and perhaps more likely, the nerve cord of the decapitated animals may respond similarly to the vertebrate spinal cord, which can initiate walking patterns in spinal-cut animals in response to applied amines (9–13). Further resolution of these issues will await identification and detailed study of the neuronal sites and receptors involved in these responses.
Sexually Dimorphic Responses.
We find a clear sexual dimorphism in response to the vertebrate dopamine D2-like receptor agonist quinpirole, and in responses to several amines in the presence of the vertebrate monoamine oxidase inhibitor hydrazaline. At high quinpirole concentrations, locomotion is stimulated similarly in males and females, but at low concentrations, males are selectively stimulated. The concentration of quinpirole for half-maximal stimulation of locomotion is 3- to 4-fold lower in males than in females (Fig. 4), most simply consistent with increased receptor affinity for quinpirole in males vs. females. Modulation of seven transmembrane-helix receptor sensitivity by phosphorylation is a hallmark of this family of receptors (49), and this modulation can change affinities for agonists (50). Changes in amine receptor sensitivity or in types of receptors have not been previously linked to sexual dimorphisms.
These results lead to the hypothesis that differences in amine receptor sensitivity or in types of receptors expressed will be associated with sexually dimorphic behaviors in Drosophila. We know of only one instance in which such behaviors have been linked to biogenic amines in Drosophila. The mutant inactive (iav) affects activity levels, experience-dependent conditioned male courtship behaviors, and octopamine levels, which are 15% of wild type (51). The locomotor inactivity in this mutant is consistent with the strong locomotor stimulation by octopamine in the decapitated flies. In apparent contrast with these results is a recent report generating octopamine-deficient flies via mutation of tyramine β-hydroxylase (52), which shows only an egg retention defect. However, as mentioned in this study, the mild phenotypes could be associated with the increased tyramine levels found in these flies, which may be capable of supplanting some, but not all, the roles of octopamine.
Why Are High Concentrations of Drugs Needed in the Decapitated Preparations?
We suspect that poor diffusion through the central nervous system accounts for the high concentrations of drugs and amines required to see responses in the decapitated preparations. Observations from our laboratory and others show that serotonin and dopamine have limited capacity for diffusion in the Drosophila central nervous system. Ddc null gynandromorphs generated using an unstable ring-X Ddc+ chromosome show that serotonin has only a very low capability for diffusion between segments in the larval nerve cord, diffusing only into the adjacent segment and across the midline (53). We recently have generated lines containing tyrosine hydroxylase transgenes resulting in altered spatial patterns of dopamine expression (Meller et al., unpublished data). Some of these lines show high levels of dopamine in some cells of the larval and adult brain lobes, but very low levels in other cells whose cell bodies are located within ≈20 μm of neuronal projections containing high levels of dopamine. This observation indicates that diffusion and uptake into these neighboring cells must be very inefficient. We suspect that the amines and amine receptor acting drugs are diffusing or are transported via the descending aminergic fibers that extend from the brain lobes into the nerve cord (19). This could explain why exogenously applied amines are having such striking effects in the decapitated preparations, by reaching regions of the nerve cord that normally are supplied with amines released by the brain.
Acknowledgments
We thank Matthew Anzivino for assistance with the behavioral measurements; W. Bender, S. Birman, C. Cronmiller, R. Palmiter, anonymous reviewers and members of our laboratory for helpful discussions, suggestions, and comments on the manuscript, and Doug Taylor and Emilie Rissman for assistance with the statistical analyses. This work was supported by National Institutes of Health Grant GM 27318 to J.H.
References
- 1.Gordon J. In: Principles of Neural Science. Kandel E R, Schwartz J H, Jessell T M, editors. New York: Elsevier Science; 1991. pp. 581–595. [Google Scholar]
- 2.Grillner G, Deliagina T, Ekeberg Ö, Manira A E, Hill R H, Lansner A, Orlovsky G N, Wallén P. Trends Neurosci. 1995;18:270–279. [PubMed] [Google Scholar]
- 3.Friesen W O. In: Neuronal and Cellular Oscillators. Jacklet J W, editor. New York: Dekker; 1989. [Google Scholar]
- 4.Grillner S, Christenson J, Brodin L, Wallén P, Hill R H, Lansner A, Ekeberg O. In: Neuronal and Cellular Oscillators. Jacket J W, editor. New York: Dekker; 1989. [Google Scholar]
- 5.Vandervorst P, Ghysen A. Nature (London) 1980;286:65–67. doi: 10.1038/286065a0. [DOI] [PubMed] [Google Scholar]
- 6.Phillis R W, Bramlage A T, Wotus C, Whittaker A, Gramates L S, Seppala D, Farahanchi F, Caruccio P, Murphey R K. Genetics. 1993;133:581–592. doi: 10.1093/genetics/133.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szebenyi A L. Anim Behav. 1969;17:641–651. [Google Scholar]
- 8.Dawkins R, Dawkins M. Anim Behav. 1976;24:739–755. [Google Scholar]
- 9.Grillner S. Acta Physiol Scand Suppl. 1969;327:1–34. [PubMed] [Google Scholar]
- 10.Grillner S. In: Neural Control of Locomotion. Herman R M, Grillner S, Stein P S G, Stuart D G, editors. New York: Plenum; 1976. pp. 351–375. [Google Scholar]
- 11.Ahlman H S, Grillner S, Udo M. Brain Res. 1971;17:393–396. doi: 10.1016/0006-8993(71)90269-1. [DOI] [PubMed] [Google Scholar]
- 12.Viala D, Buser P. Brain Res. 1969;12:437–443. doi: 10.1016/0006-8993(69)90011-0. [DOI] [PubMed] [Google Scholar]
- 13.Barbeau H, Rossignol S. Brain Res. 1991;546:250–260. doi: 10.1016/0006-8993(91)91489-n. [DOI] [PubMed] [Google Scholar]
- 14.Sombati S, Hoyle G. J Neurobiol. 1984;15:481–506. doi: 10.1002/neu.480150607. [DOI] [PubMed] [Google Scholar]
- 15.Stevenson P, Kutsch W. Naturwissenschaften. 1986;73:741–743. [Google Scholar]
- 16.Claassen D E, Kammer A E. J Neurobiol. 1986;17:1–14. doi: 10.1002/neu.480170102. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein R S, Camhi J M. J Comp Physiol A. 1991;168:103–112. doi: 10.1007/BF00217108. [DOI] [PubMed] [Google Scholar]
- 18.Cooper J R, Bloom F E, Roth R H, editors. The Biochemical Basis of Neuropharmacology. London: Oxford Univ. Press; 1991. [Google Scholar]
- 19.Nassel D R, Elekes K. Cell Tiss Res. 1992;267:147–167. doi: 10.1007/BF00318701. [DOI] [PubMed] [Google Scholar]
- 20.White K, Vallés A M. In: Molecular Bases of Neural Development. Edelman G M, Gall W E, Cowan W M, editors. New York: Wiley; 1985. pp. 547–563. [Google Scholar]
- 21.Beall C, Hirsh J. Genes Dev. 1987;1:510–520. doi: 10.1101/gad.1.5.510. [DOI] [PubMed] [Google Scholar]
- 22.Cournil I, Helluy S M, Beltz B S. J Comp Neurol. 1994;344:455–469. doi: 10.1002/cne.903440308. [DOI] [PubMed] [Google Scholar]
- 23.Beltz B S, Pontes M, Helluy S M, Kravitz E A. J Neurobiol. 1990;21:521–542. doi: 10.1002/neu.480210402. [DOI] [PubMed] [Google Scholar]
- 24.Jackson D M, Westlind-Danielsson A. Pharmacol Ther. 1994;64:291–369. doi: 10.1016/0163-7258(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 25.Saudou F, Hen R. Neurochem Int. 1994;25:503–532. doi: 10.1016/0197-0186(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 26.Sugamori K S, Tol H H M V, Niznik H B. In: Dopamine Receptors and Transporters: Pharmacology, Structure, and Function. Niznik H B, editor. New York: Dekker; 1994. pp. 103–132. [Google Scholar]
- 27.Treherne J E. In: Insect Neurobiology. Treherne J E, editor. Amsterdam: North–Holland; 1974. pp. 187–244. [Google Scholar]
- 28.O’Dell K M, Armstrong J D, Yang M Y, Kaiser K. Neuron. 1995;15:55–61. doi: 10.1016/0896-6273(95)90064-0. [DOI] [PubMed] [Google Scholar]
- 29.Ferveur J F, Stortkuhl K F, Stocker R F, Greenspan R J. Science. 1995;267:902–905. doi: 10.1126/science.7846534. [DOI] [PubMed] [Google Scholar]
- 30.Booker R, Quinn W G. Proc Nat Acad Sci USA. 1981;78:3940–3944. doi: 10.1073/pnas.78.6.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.SAS Institute. SAS/STAT User’s Guide. Cary, NC: SAS Institute; 1989. [Google Scholar]
- 32.Sokal R R, Rohlf F J, editors. Biometry. New York: Freeman; 1995. [Google Scholar]
- 33.Rice W R. Evolution. 1989;43:223–225. doi: 10.1111/j.1558-5646.1989.tb04220.x. [DOI] [PubMed] [Google Scholar]
- 34.Dewhurst S A, Croker S G, Ikeda K, McCaman R E. Comp Biochem Physiol B. 1972;43:975–981. doi: 10.1016/0305-0491(72)90241-6. [DOI] [PubMed] [Google Scholar]
- 35.Brown C S, Nestler C. In: Comprehensive Insect Physiology, Biochemistry, and Pharmacology. Kerkut G A, Gilbert L I, editors. New York: Pergamon; 1985. pp. 435–496. [Google Scholar]
- 36.Gotzes F, Balfanz S, Baumann A. Recept Channels. 1994;2:131–141. [PubMed] [Google Scholar]
- 37.Sugamori K S, Demchyshyn L L, McConkey F, Forte M A, Niznik H B. FEBS Lett. 1995;362:131–138. doi: 10.1016/0014-5793(95)00224-w. [DOI] [PubMed] [Google Scholar]
- 38.Feng G, Hannan F, Reale V, Hon Y Y, Kousky C T, Evans P D, Hall L M. J Neurosci. 1996;16:3925–3933. doi: 10.1523/JNEUROSCI.16-12-03925.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han K-A, Millar N S, Grotewiel M S, Davis R L. Neuron. 1996;16:1127–1135. doi: 10.1016/s0896-6273(00)80139-7. [DOI] [PubMed] [Google Scholar]
- 40.Saudou F, Amlaiky N, Plassat J L, Borrelli E, Hen R. EMBO J. 1990;9:3611–3617. doi: 10.1002/j.1460-2075.1990.tb07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arakawa S, Gocayne J D, McCombie W R, Urquhart D A, Hall L M, Fraser C M, Venter J C. Neuron. 1990;4:343–354. doi: 10.1016/0896-6273(90)90047-j. [DOI] [PubMed] [Google Scholar]
- 42.Vanden Broeck J, Vulsteke V, Huybrechts R, De Loof A. J Neurochem. 1995;6455:2387–2395. doi: 10.1046/j.1471-4159.1995.64062387.x. [DOI] [PubMed] [Google Scholar]
- 43.Robb S, Cheek T R, Hannan F L, Hall L M, Midgley J M, Evans P D. EMBO J. 1994;13:1325–1330. doi: 10.1002/j.1460-2075.1994.tb06385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kokay I C, Mercer A R. Brain Res. 1996;706:47–56. doi: 10.1016/0006-8993(95)01179-x. [DOI] [PubMed] [Google Scholar]
- 45.Eilam D, Talangbayan H, Canaran G, Szechtman H. Psychopharmacology. 1992;106:447–454. doi: 10.1007/BF02244813. [DOI] [PubMed] [Google Scholar]
- 46.Morelli M, Chiara G D. Eur J Pharmacol. 1985;117:179–185. doi: 10.1016/0014-2999(85)90602-8. [DOI] [PubMed] [Google Scholar]
- 47.Waddington J L, Daly S A. In: D1:D2 Dopamine Receptor Interactions. Waddington J L, editor. London: Academic; 1993. pp. 51–78. [Google Scholar]
- 48.Venturini G, Stocchi F, Margotta V, Ruggieri S, Bravi D. Neuropharmacology. 1989;28:1377–1382. doi: 10.1016/0028-3908(89)90013-0. [DOI] [PubMed] [Google Scholar]
- 49.Premont R T, Inglese J, Lefkowitz R J. FASEB J. 1995;9:175–182. doi: 10.1096/fasebj.9.2.7781920. [DOI] [PubMed] [Google Scholar]
- 50.Liggett S B, Ostrowski J, Chesnut L C, Kurose H, Raymond J R, Caron M G, Lefkowitz R J. J Biol Chem. 1992;267:4740–4746. [PubMed] [Google Scholar]
- 51.O’Dell K M. Behav Genet. 1994;24:381–388. doi: 10.1007/BF01067539. [DOI] [PubMed] [Google Scholar]
- 52.Monastirioti M, Linn C E, White K. J Neurosci. 1996;16:3900–3911. doi: 10.1523/JNEUROSCI.16-12-03900.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Valles A M, White K. J Neurosci. 1990;10:3646–3656. doi: 10.1523/JNEUROSCI.10-11-03646.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]