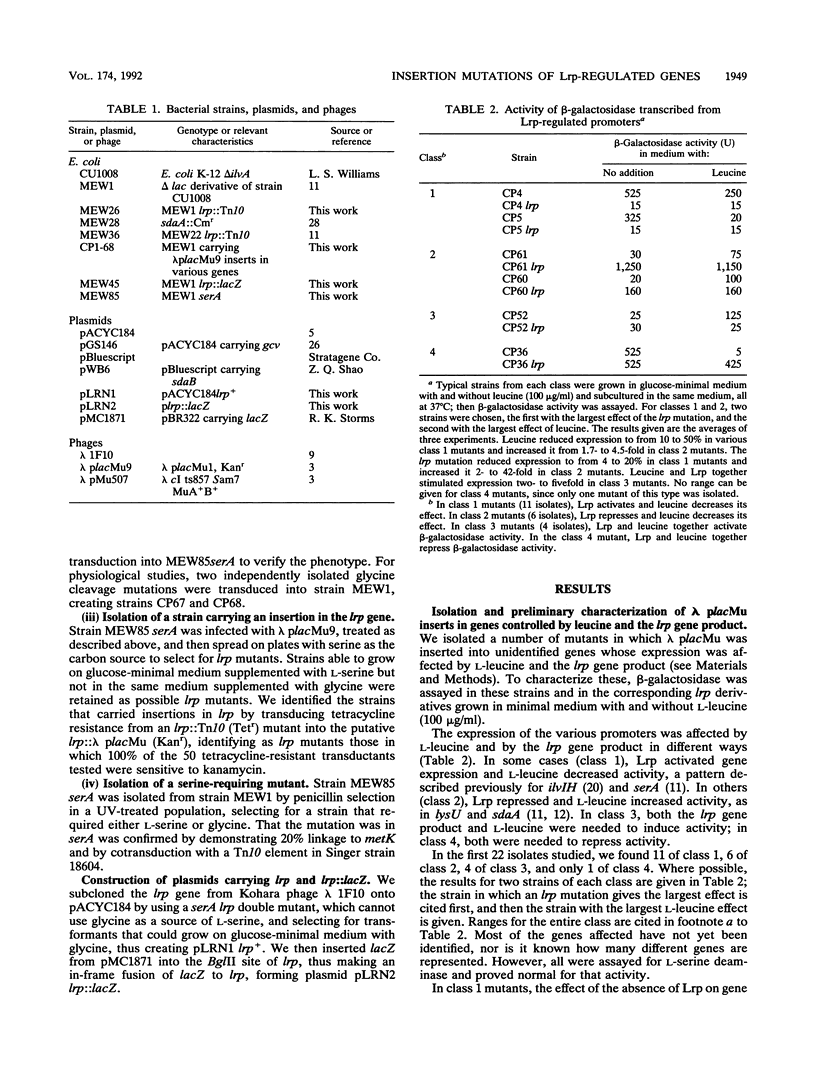
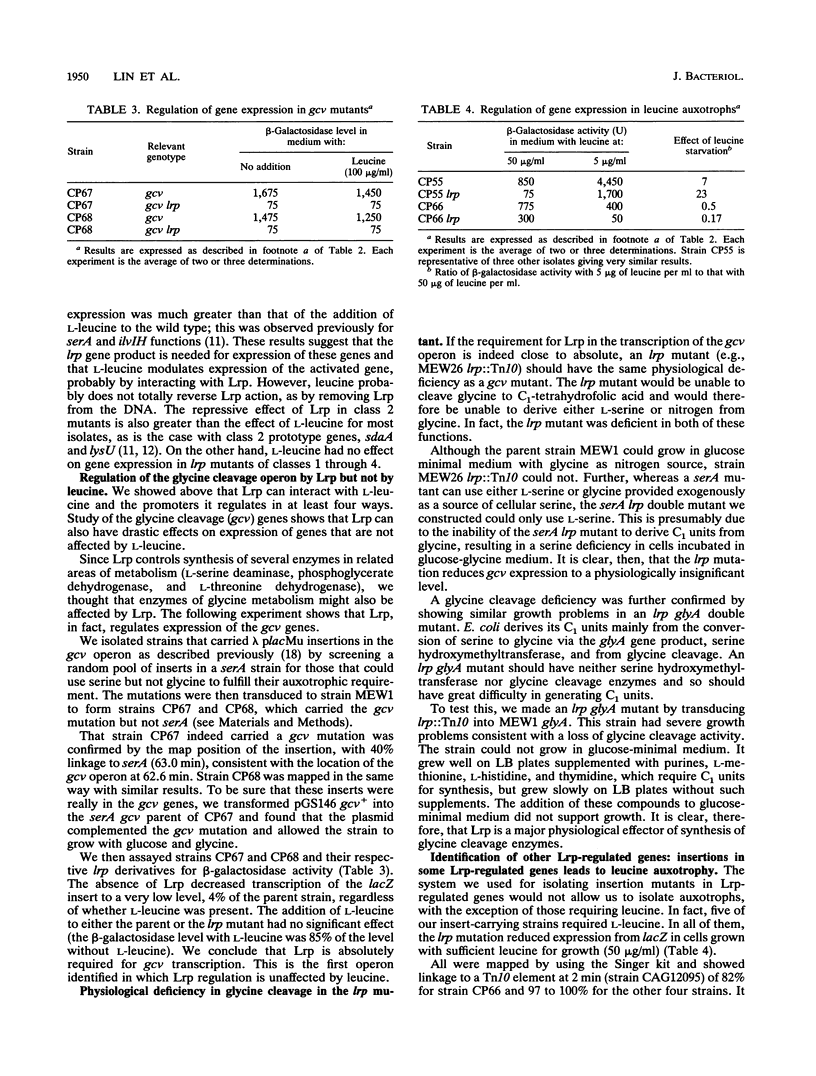
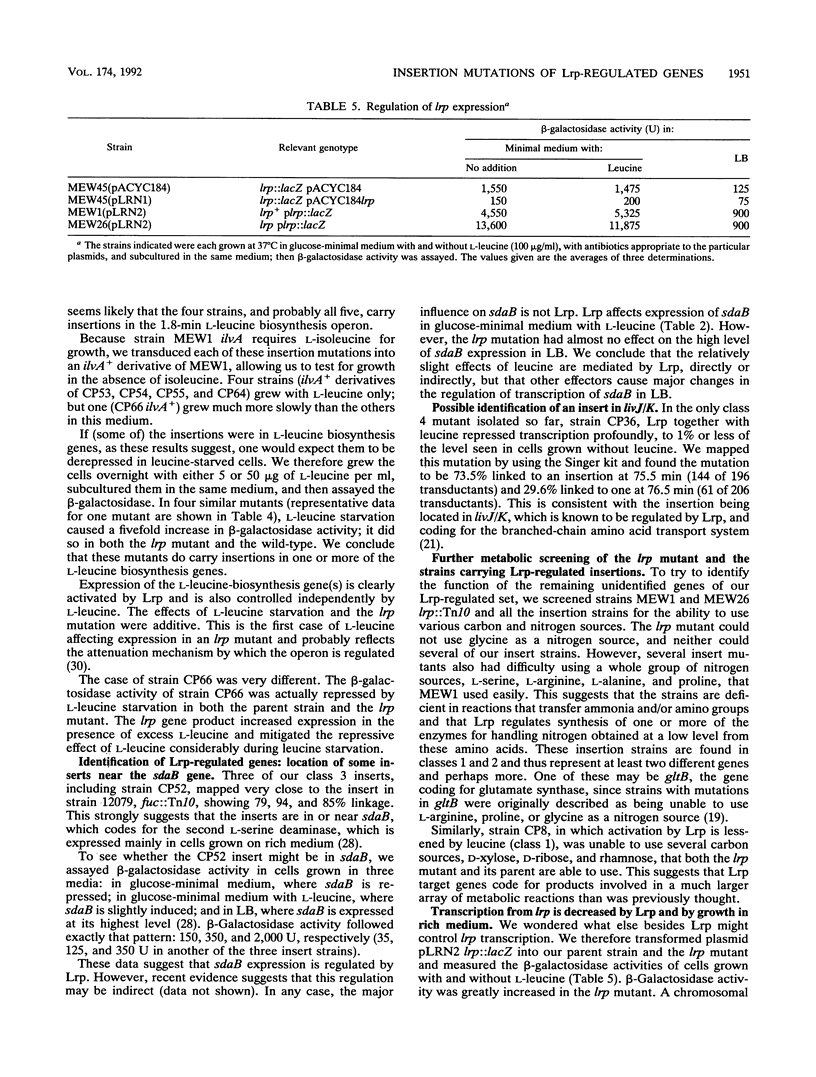
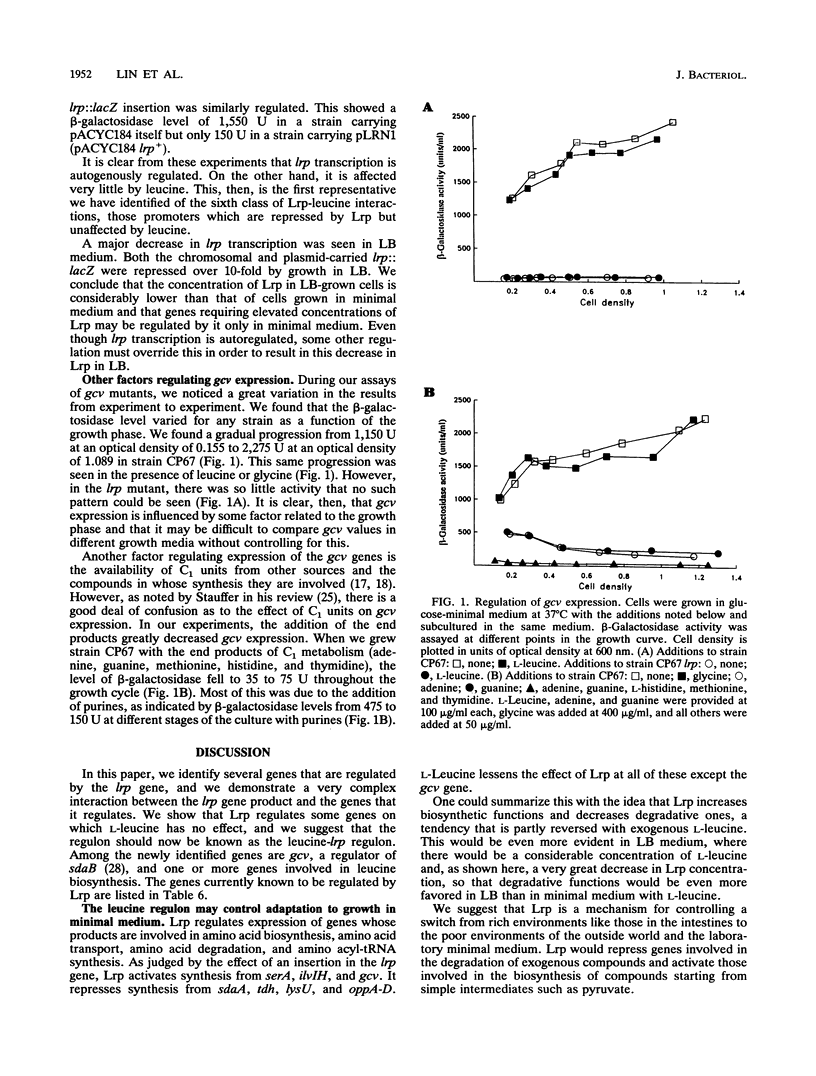
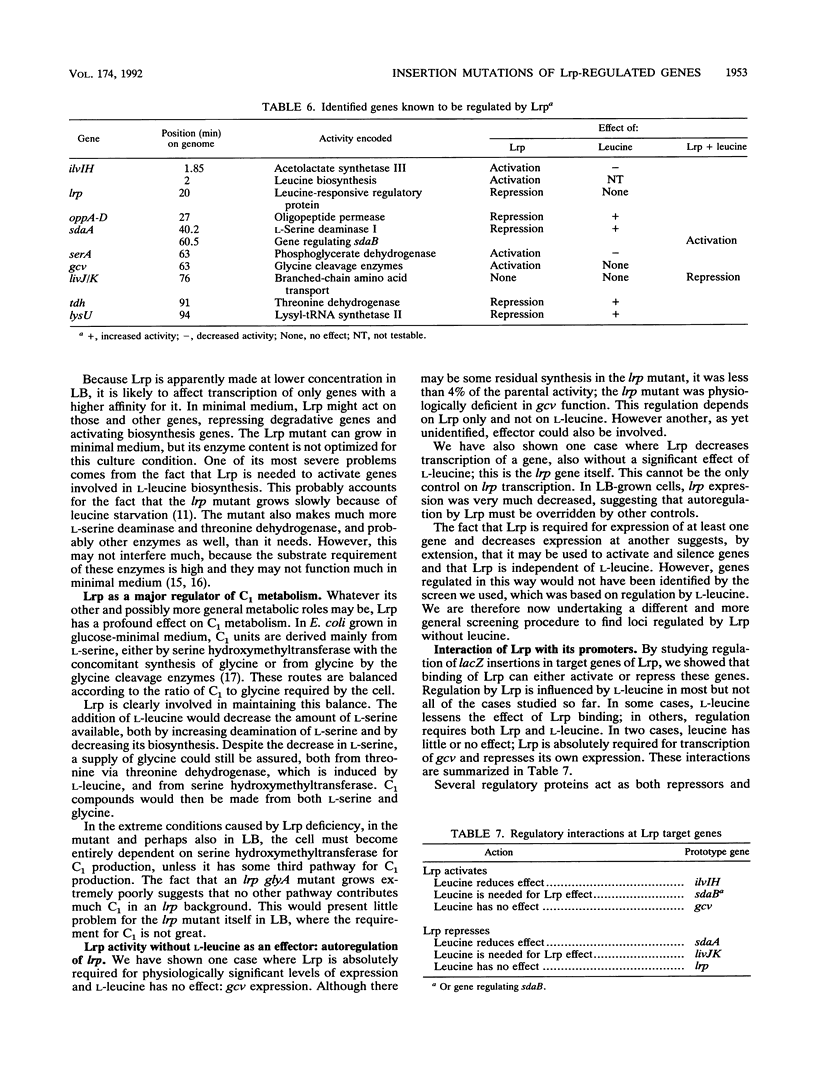
Abstract
The leucine regulon coordinates the expression of several Escherichia coli genes according to the presence of exogenous leucine, which interacts with the lrp gene product, Lrp. We isolated and characterized 22 strains with lambda placMu insertions in Lrp-regulated genes. Lrp and leucine influenced gene expression in a surprising variety of ways. We identified two genes that are regulated by Lrp and not affected by L-leucine. We therefore rename this the leucine-lrp regulon. Genes coding for glycine cleavage and leucine biosynthesis enzymes have been identified as members of the leucine-lrp regulon. We suggest that the lrp gene product activates genes needed for growth in minimal medium, and we show that the gene is repressed by its own product and is highly repressed during growth in rich medium.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Mizuno T. Phosphorylation of a bacterial activator protein, OmpR, by a protein kinase, EnvZ, stimulates the transcription of the ompF and ompC genes in Escherichia coli. FEBS Lett. 1990 Feb 12;261(1):19–22. doi: 10.1016/0014-5793(90)80626-t. [DOI] [PubMed] [Google Scholar]
- Andrews J. C., Short S. A. opp-lac Operon fusions and transcriptional regulation of the Escherichia coli trp-linked oligopeptide permease. J Bacteriol. 1986 Feb;165(2):434–442. doi: 10.1128/jb.165.2.434-442.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer E., Silhavy T. J., Weinstock G. M. Transposable lambda placMu bacteriophages for creating lacZ operon fusions and kanamycin resistance insertions in Escherichia coli. J Bacteriol. 1985 Jun;162(3):1092–1099. doi: 10.1128/jb.162.3.1092-1099.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindle P. K., Holland J. P., Willett C. E., Innis M. A., Holland M. J. Multiple factors bind the upstream activation sites of the yeast enolase genes ENO1 and ENO2: ABFI protein, like repressor activator protein RAP1, binds cis-acting sequences which modulate repression or activation of transcription. Mol Cell Biol. 1990 Sep;10(9):4872–4885. doi: 10.1128/mcb.10.9.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drlica K., Rouviere-Yaniv J. Histonelike proteins of bacteria. Microbiol Rev. 1987 Sep;51(3):301–319. doi: 10.1128/mr.51.3.301-319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesman D., Best L., Tatchell K. The role of RAP1 in the regulation of the MAT alpha locus. Mol Cell Biol. 1991 Feb;11(2):1069–1079. doi: 10.1128/mcb.11.2.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C. J., Walker G. C. DNA-damaging agents stimulate gene expression at specific loci in Escherichia coli. Proc Natl Acad Sci U S A. 1980 May;77(5):2819–2823. doi: 10.1073/pnas.77.5.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Kolodrubetz D., Schleif R. Identification of araC protein and two-dimensional gels, its in vivo instability and normal level. J Mol Biol. 1981 Jun 15;149(1):133–139. doi: 10.1016/0022-2836(81)90265-5. [DOI] [PubMed] [Google Scholar]
- NEWMAN E. B., MAGASANIK B. THE RELATION OF SERINE--GLYCINE METABOLISM TO THE FORMATION OF SINGLE-CARBON UNITS. Biochim Biophys Acta. 1963 Nov 15;78:437–448. doi: 10.1016/0006-3002(63)90905-3. [DOI] [PubMed] [Google Scholar]
- Newman E. B., Kapoor V. In vitro studies on L-serine deaminase activity of Escherichia coli K12. Can J Biochem. 1980 Nov;58(11):1292–1297. doi: 10.1139/o80-173. [DOI] [PubMed] [Google Scholar]
- Newman E. B., Kapoor V., Potter R. Role of L-threonine dehydrogenase in the catabolism of threonine and synthesis of glycine by Escherichia coli. J Bacteriol. 1976 Jun;126(3):1245–1249. doi: 10.1128/jb.126.3.1245-1249.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platko J. V., Willins D. A., Calvo J. M. The ilvIH operon of Escherichia coli is positively regulated. J Bacteriol. 1990 Aug;172(8):4563–4570. doi: 10.1128/jb.172.8.4563-4570.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quay S. C., Dick T. E., Oxender D. L. Role of transport systems in amino acid metabolism: leucine toxicity and the branched-chain amino acid transport systems. J Bacteriol. 1977 Mar;129(3):1257–1265. doi: 10.1128/jb.129.3.1257-1265.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricca E., Aker D. A., Calvo J. M. A protein that binds to the regulatory region of the Escherichia coli ilvIH operon. J Bacteriol. 1989 Mar;171(3):1658–1664. doi: 10.1128/jb.171.3.1658-1664.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S. C., Shields G. C., Steitz T. A. Crystal structure of a CAP-DNA complex: the DNA is bent by 90 degrees. Science. 1991 Aug 30;253(5023):1001–1007. doi: 10.1126/science.1653449. [DOI] [PubMed] [Google Scholar]
- Singer M., Baker T. A., Schnitzler G., Deischel S. M., Goel M., Dove W., Jaacks K. J., Grossman A. D., Erickson J. W., Gross C. A. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol Rev. 1989 Mar;53(1):1–24. doi: 10.1128/mr.53.1.1-24.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer L. T., Plamann M. D., Stauffer G. V. Cloning and characterization of the glycine-cleavage enzyme system of Escherichia coli. Gene. 1986;44(2-3):219–226. doi: 10.1016/0378-1119(86)90185-x. [DOI] [PubMed] [Google Scholar]
- Stirling C. J., Szatmari G., Stewart G., Smith M. C., Sherratt D. J. The arginine repressor is essential for plasmid-stabilizing site-specific recombination at the ColE1 cer locus. EMBO J. 1988 Dec 20;7(13):4389–4395. doi: 10.1002/j.1460-2075.1988.tb03338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Newman E. B. A novel L-serine deaminase activity in Escherichia coli K-12. J Bacteriol. 1991 Apr;173(8):2473–2480. doi: 10.1128/jb.173.8.2473-2480.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsung K., Brissette R. E., Inouye M. Enhancement of RNA polymerase binding to promoters by a transcriptional activator, OmpR, in Escherichia coli: its positive and negative effects on transcription. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5940–5944. doi: 10.1073/pnas.87.15.5940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuan L. R., D'Ari R., Newman E. B. The leucine regulon of Escherichia coli K-12: a mutation in rblA alters expression of L-leucine-dependent metabolic operons. J Bacteriol. 1990 Aug;172(8):4529–4535. doi: 10.1128/jb.172.8.4529-4535.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessler S. R., Calvo J. M. Control of leu operon expression in Escherichia coli by a transcription attenuation mechanism. J Mol Biol. 1981 Jul 15;149(4):579–597. doi: 10.1016/0022-2836(81)90348-x. [DOI] [PubMed] [Google Scholar]
- Willins D. A., Ryan C. W., Platko J. V., Calvo J. M. Characterization of Lrp, and Escherichia coli regulatory protein that mediates a global response to leucine. J Biol Chem. 1991 Jun 15;266(17):10768–10774. [PubMed] [Google Scholar]



