Abstract
Glutamate, the major excitatory neurotransmitter in the mammalian central nervous system, is transported into and stored in synaptic vesicles. We have purified to apparent homogeneity a protein from brain cytosol that inhibits glutamate and γ-aminobutyric acid uptake into synaptic vesicles and have termed this protein “inhibitory protein factor” (IPF). IPF refers to three distinct proteins with relative molecular weights of 138,000 (IPF α), 135,000 (IPF β), and 132,000 (IPF γ), respectively. Gel filtration and sedimentation data suggest that all three proteins share an elongated structure, identical Stokes radius (60 Å), and identical sedimentation coefficient (4.3 S). Using these values and a partial specific volume of 0.716 ml/g, we determined the native molecular weight for IPF α to be 103,000. Partial sequence analysis shows that IPF α is derived from α fodrin, a protein implicated in several diverse cellular activities. IPF α inhibits ATP-dependent glutamate uptake into purified synaptic vesicles with an IC50 of ≈26 nM, while showing no ability to inhibit ATP-independent uptake at concentrations up to 100 nM. Moreover, IPF α inhibited neither norepinephrine uptake into chromaffin vesicles nor Na+-dependent glutamate uptake into synaptosomes. However, IPF α inhibited uptake of γ-aminobutyric acid into synaptic vesicles derived from spinal cord, suggesting that inhibition may not be limited to glutamatergic systems. We propose that IPF could be a novel component of a presynaptic regulatory system. Such a system might modulate neurotransmitter accumulation into synaptic vesicles and thus regulate the overall efficacy of neurotransmission.
Keywords: regulation, fodrin, transport, presynaptic, neurotransmission
Glutamate is now widely accepted as the major excitatory neurotransmitter in the central nervous system of all vertebrates (1–6). Abnormalities in glutamatergic synaptic transmission have been implicated in many neuropathologies, including certain seizures, ischemia-induced neuronal death, schizophrenia, Alzheimer disease, Parkinson disease, and Huntington disease (7–11). Excessive release of glutamate into the synaptic cleft is believed to be a common underlying basis for many of these disease states. There is also evidence that some glutamate receptors such as the N-methyl-d-aspartate and metabotropic receptors may be involved in neuronal plasticity (12–14).
Evidence accumulated for the last decade strongly supports the notion that glutamatergic neurotransmission occurs through an exocytotic process involving the interaction of glutamate-containing synaptic vesicles with the plasma membrane of the presynaptic ending. In support of this is the observation that glutamate is taken up into purified, isolated synaptic vesicles in an ATP-dependent manner (15–20), consistent with immunocytochemical evidence that glutamate is concentrated in synaptic vesicles that are distinct from γ-aminobutyric acid (GABA) vesicles (21). Biochemical evidence (22) also suggested that high concentrations of glutamate are accumulated in brain synaptic vesicles in vivo. Studies by Nicholls and coworkers (23, 24) indicate that the exocytotic pool of glutamate originates from a noncytoplasmic site within the nerve terminal. Moreover, Kish and Ueda (25) provided evidence that vesicular glutamate is released in a calcium-dependent manner from permeabilized synaptosomes. Recently, Wilson and coworkers (26) have shown that calcium-dependent release of glutamate involves SNAP-25 and synaptotagmin, components thought to play an important role in exocytosis. This wealth of data clearly demonstrates that synaptic vesicles are the storage site of the glutamate to be released from nerve terminals.
The vesicular glutamate uptake system has several distinctive properties that distinguish it from the cellular glutamate re-uptake system present in the plasma membrane. It is stringently specific for glutamate, has a relatively high Km, and requires low concentrations of chloride for optimal activity (16, 19). The driving force for glutamate uptake is provided by an electrochemical proton gradient generated by a V-type H+-ATPase (vacuolar-type proton-translocating ATPase) in the synaptic vesicle membrane (16–18, 27). The precise mechanism by which the glutamate transporter uses this proton gradient to drive glutamate uptake is not fully understood; however, compounds that interfere with the formation of such gradients have a marked inhibitory effect on glutamate transport (16–19, 28).
It has been proposed that glutamate uptake into synaptic vesicles represents the critical step in diverting glutamate away from the metabotropic pathway and toward the neurotransmitter pathway (29). In biological systems, such branching points in metabolic pathways are often sites of regulation. Thus, it is conceivable that the vesicular glutamate uptake system may be regulated under normal physiological conditions. Although many studies have focused on changes associated with postsynaptic glutamate receptors, few have addressed presynaptic regulation of glutamatergic neurotransmission at the level of vesicular transport. Alterations in such a regulatory system could cause the abnormalities in glutamatergic neurotransmission implicated in the variety of central nervous system disorders mentioned earlier.
We have previously described an endogenous, proteinaceous factor in the cytosol from nerve endings, which inhibits vesicular glutamate uptake (30), raising the possibility that such a substance might have a regulatory role in vivo. In this work, we report the purification of a 138-kDa protein to apparent homogeneity, which inhibits glutamate and GABA storage in synaptic vesicles with high potency. This protein is part of a group of three structurally related proteins, referred to as inhibitory protein factor (IPF) αβγ. Partial sequence analysis suggests that IPF may be derived from the well characterized structural protein α fodrin. In addition to the isolation, some of the physicochemical properties of IPF are discussed, as well as possible modes of action and a hypothesized physiological role.
MATERIALS AND METHODS
Materials.
Polyethyleneimine (PAE)-1000 anion-exchanger was purchased from Amicon. HTP hydroxyapatite was from Bio-Rad. Superdex S200 26/60 and Mono Q 5/5 columns were purchased from Pharmacia. [3H]Glutamate (50 Ci/mmol; 1 Ci = 37 GBq) was purchased from Amersham. Cyto Scint ES scintillation fluid was from ICN. Whatman GFC filters were purchased from VWR Scientific. Ammonium sulfate was from Mallinckrodt. Protein was quantified with the Coomassie Protein Assay Kit from Pierce, with BSA as standard. All other chemicals and chromatography media were purchased from Sigma.
Preparation of Synaptic Vesicles and Synaptosomes.
All of the following procedures were performed on ice or in a 4°C cold room unless noted. All the centrifugal force values given refer to the maximal forces created at the bottom of centrifuge tubes at the various rotation speeds. Bovine synaptic vesicles were prepared by a slight modification of the procedure described by Tabb et al. (27). The discontinuous sucrose gradients did not contain the 0.8 M sucrose layer, and centrifugation was carried out in a Beckman Type 45Ti rotor at 35,000 rpm (140,000 × g) for 2 hr. Vesicles were stored in liquid nitrogen where glutamate uptake activity was unchanged for at least 3 months. Bovine synaptosomes used in Fig. 5 were collected during the preparation of synaptic vesicles (27). Synaptosomes were resuspended in normal Krebs–Ringer solution (0.15 M NaCl/6.2 mM KCl/1.2 mM Na2HPO4/1.2 mM MgSO4/10 mM glucose/20 mM Tris·Hepes, pH 7.4) or low Na+ Krebs-Ringer solution (substituting 0.15 M choline chloride for NaCl) before the assay.
Figure 5.
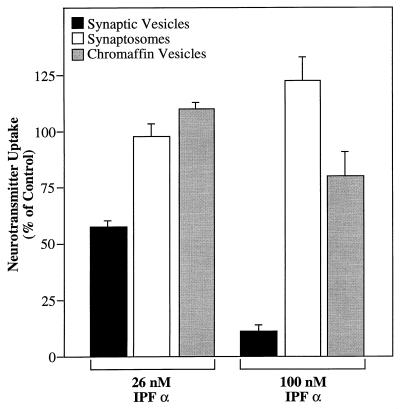
Specificity of the inhibitory effect produced by IPF α. Bovine synaptic vesicles (50 μg of protein) were suspended in the glutamate uptake assay medium as described in the text. Bovine synaptosomes (35 μg of protein) were suspended in 0.12 ml of Krebs-Ringer solution containing 1 mM spermine and 1 μM [3H]glutamate (1.67 Ci/mmol) in the presence of Na+ (150 mM) or choline (150 mM). Bovine chromaffin vesicles (45 μg of protein) were suspended in 0.12 ml of a solution containing 0.3 M sucrose, 1 mM spermine, 10 mM MgSO4, 5 mM ATP, 10 mM Hepes (pH 7.0), and 50 μM [3H]norepinephrine (0.017 Ci/mmol) with or without 1 μM reserpine. Each membrane mixture also contained either 0, 26, or 100 nM IPF α. Glutamate uptake was allowed to proceed for 10 min at 30°C and 30 min at 37°C for norepinephrine uptake. Reaction was terminated by filtration as described in in the text. Values for the percentage of control were calculated relative to the specific uptake activity obtained in the absence of IPF α. Uptake into synaptic vesicles is represented by ATP-dependent uptake, that into synaptosomes by Na+-dependent uptake and that into chromaffin vesicles by reserpine-sensitive uptake.
Standard Assay of IPF.
The uptake of [3H]glutamate into synaptic vesicles was assayed using a modification of the filtration procedure described in ref. 31. Briefly, synaptic vesicles (30–50 μg of protein) were suspended in 120 μl of an incubation medium consisting of 0.23 M sucrose, 4 mM KCl, 4 mM MgSO4, 2 mM aspartate, 10 mM methionine sulfoximine, 1 mM spermine, ± 2 mM ATP, ± IPF sample 10 mM Hepes (pH 7.4), and 50 μM [3H]glutamate (specific activity, 0.017 Ci/mmol). Glutamate uptake was initiated by transferring the mixtures from ice to a 30°C water bath, and the uptake reaction was allowed to proceed for 10 min. Baseline ATP-dependent glutamate uptake activity was calculated as the glutamate taken up in the presence of ATP minus that taken up in the absence of ATP. Throughout this work, glutamate uptake activity refers to the ATP-dependent portion, which was typically greater than 90% of the total. One unit of the IPF was defined as the amount of protein required to inhibit 50% of ATP-dependent glutamate uptake over a period of 10 min at 30°C.
Preparation of the Crude Cellular Extract from Bovine Brain.
Typically, 25 calf brains were obtained fresh from a local slaughterhouse, and the cerebellum, brainstem, and excess white matter were removed to yield 5,500–6,000 g of cerebral tissue. A Waring blender was used to mince 300 g of cortex at a time in 800 ml of lysing buffer [1 mM phenylmethylsulfonyl fluoride (PMSF)/1 mM EDTA/5 mM 2-mercaptoethanol/6 mM Tris·HCl, pH 8.3]. The blended material was then diluted to ≈40 liters in lysing buffer and homogenized to smoothness by passing through a large, continuous-flow homogenizer. The entire suspension was then centrifuged at 13,000 rpm (27,300 × g) for 15 min in a Sorvall GSA rotor. The resulting supernatant (≈25 liters) was concentrated to 10 liters in an Amicon spiral ultra-concentrator equipped with a S1Y30 cartridge (30,000 molecular weight cutoff) before further fractionation.
RESULTS
Purification of IPF.
The IPF was purified to apparent homogeneity by the steps summarized in Table 1, indicating a 1,160 to 1,280-fold purification for IPF α and IPF β, respectively.
Table 1.
Purification of IPF from calf brain cytosol
| Fraction | Total protein, mg | IC50, mg/0.12 ml | Total activity, units | Yield, % | Specific activity, units/mg | Purification, -fold |
|---|---|---|---|---|---|---|
| Cytosol | 69,960 | 0.50 | 139,920 | 100 | 2 | 1 |
| 45% AS ppt | 26,920 | 0.25 | 107,680 | 77 | 4 | 2 |
| 0.5 M PAE | 6,952 | 0.081 | 85,827 | 61 | 12 | 6 |
| 0.05 M HTP | 1,824 | 0.040 | 45,600 | 33 | 25 | 13 |
| 0.3 M Yellow-86 | 58 | 0.011 | 5,273 | 3.8 | 91 | 46 |
| Superdex peak | 23 | 0.0050 | 4,600 | 3.3 | 200 | 100 |
| Sucrose peak | 2.5 | 0.0011 | 2,273 | 1.6 | 909 | 455 |
| Mono Q IPF α | 0.25 | 0.00043 | 581 | 0.42 | 2,326 | 1,160 |
| Mono Q IPF β | 0.25 | 0.00039 | 641 | 0.46 | 2,564 | 1,280 |
Step 1: Ammonium sulfate precipitation.
The crude cellular extract (≈10 liters) was adjusted to 45% saturation with ammonium sulfate and incubated for 130 min. The precipitate was collected by centrifugation for 15 min at 13,000 rpm (27,300 × g), resuspended to 2.5 liters in lysing buffer and dialyzed overnight against 55 liters of same. The dialyzed sample was then clarified by centrifuging for 70 min at 45,000 rpm (235,400 × g) in a Beckman Type 45Ti rotor. The resulting supernatant (2.6 liters, 24,000 mg of protein) was generally stored overnight at −20°C before anion-exchange chromatography.
Step 2: Anion-exchange chromatography.
One half of the 45% ammonium sulfate precipitate at a time (≈1.3 liters) was loaded onto a PAE-1000 column (7.5 cm × 32 cm) equilibrated with lysing buffer, at a flow rate of 50 ml/min. After collection of the flow-through fraction, bound protein was eluted with 2 liters each of 0.2, 0.5, and 1.0 M NaCl dissolved in the column buffer. The 0.5 M NaCl eluate (1.5 liters) was dialyzed overnight against 58 liters of HAP column buffer solution (1 mM MgCl2/0.2 mM PMSF/10 mM Tris·maleate, pH 8.0). The 0.5 M NaCl eluate was usually fractionated on hydroxyapatite immediately after dialysis.
Step 3: Hydroxyapatite column chromatography.
This step was typically run twice, with each run using one of the two 0.5 M NaCl eluates obtained from the PAE-1000 column. The dialyzed PAE 0.5 M eluate (1.6 liters, 3,500 mg of protein) was applied to a hydroxyapatite column (7.5 cm × 9 cm) equilibrated with HTP column buffer at a flow rate of 20 ml/min. Bound protein was eluted with increasing steps of potassium phosphate (0.01, 0.05, 0.1, and 1 M) dissolved in HTP column buffer. The 0.05 M eluate was collected and later combined with the same fraction obtained from the second column run. The combined HTP 0.05 M eluates were adjusted to 80% saturation with ammonium sulfate, and the precipitates were collected and resuspended to 200 ml in yellow column buffer (1 mM EDTA/0.2 mM PMSF/10 mM Tris·maleate, pH 7.0). This was dialyzed overnight against two 18-liter changes of the same.
Step 4: Reactive Yellow-86 chromatography.
The dialyzed 0.05 M phosphate eluate from the hydroxyapatite column (250 ml, 1,800 mg of protein) was loaded onto a Reactive Yellow-86 agarose column (4.5 cm × 22 cm) equilibrated with yellow column buffer. Approximately 95% of loaded protein does not bind to the column and is collected in the flow-through. Bound protein is eluted with successive steps of 0.06, 0.3, and 1 M NaCl dissolved in column buffer, at a flow rate of 14 ml/min. The 0.3 M NaCl eluate (250 ml, 60 mg of protein) was adjusted to 80% saturation with ammonium sulfate, and the precipitate was collected as previously described. The precipitate was resuspended to 10 ml in yellow column buffer and dialyzed overnight against two 5-liter changes of the same.
Step 5: Gel filtration on Superdex S200.
The dialyzed Yellow-86 0.3 M NaCl eluate (10 ml, 18–20 mg of protein) was applied to a Superdex S-200 26/60 column (2.6 cm × 62 cm) equilibrated with a solution containing 75 mM KCl, 1 mM EDTA, 0.2 mM PMSF, and 10 mM Tris·maleate (pH 7.0). The column was run at 1 ml/min, and 6-ml fractions were collected (60 total). Typically, fractions 23–30 were pooled, and an 80% ammonium sulfate precipitate was collected as previously described. The precipitate was resuspended in 2 ml of a solution containing 1 mM EDTA, 0.2 mM PMSF, and 10 mM Tris·maleate (pH 7.0) and dialyzed against 4 liters of the same for 4 hr just before sucrose gradient centrifugation.
The estimated Stokes radius was determined by using plots of (−logKav)1/2 vs. the Stokes radius of standard proteins (ferritin, 79 Å; BSA, 35 Å; and myoglobin, 17 Å) according to ref. 32).
Step 6: Sucrose density gradient centrifugation.
The dialyzed ammonium sulfate precipitate from the Superdex S-200 column (2.5 ml, 20–25 mg of protein) was layered onto two 36-ml 5–20% sucrose gradients developed in a solution containing 50 mM NaCl, 1 mM EDTA, 0.2 mM PMSF, and 10 mM Hepes (pH 7.4). Gradients were centrifuged for 43 hr at 28,000 rpm (140,000 × g) in a Beckman SW28 rotor. A gradient containing catalase (11.3 S), BSA (4.3 S), and myoglobin (2.1 S) was also run in parallel to determine the sedimentation coefficients in Table 2 (33). Typically, 36 1-ml fractions were collected from each gradient by puncturing the tube bottoms.
Table 2.
Physicochemical properties of IPF
Step 7: HPLC anion-exchange chromatography.
Peak inhibitory fractions from the sucrose gradients (typically fractions 22–26, ≈2 mg of protein) were pooled and applied to a Pharmacia Mono Q 5/5 anion-exchange column equilibrated with a solution containing 75 mM NaCl, 1 mM EDTA, 0.2 mM PMSF, and 20 mM Tris·HCl (pH 7.6). Bound protein was eluted with a linear NaCl gradient (75–538 mM) developed over 60 min at a flow rate of 1 ml/min. Fractions were collected (60 × 1 ml), and 10-μl aliquots were assayed for inhibitory activity. Controls were performed by assaying aliquots from an identical gradient run in the absence of added protein.
Typical results of the procedure used to purify IPF α and IPF β are shown in Table 1 and in Figs. 1 and 2. Hydroxyapatite chromatography at pH 8.0 in the presence of Mg2+ proved to be a critical step during purification. Under these conditions, virtually all of the IPF was found in the 0.05 M phosphate eluate (peak 1) and resolved from a second peak of inhibitory activity present in the 0.1 M eluate (peak 2). This accounted for the 50% loss of total inhibitory activity seen at this step. The activity in peak 2 correlated with a 73-kDa protein that may represent a proteolytic digestion product of IPF (unpublished observation).
Figure 1.
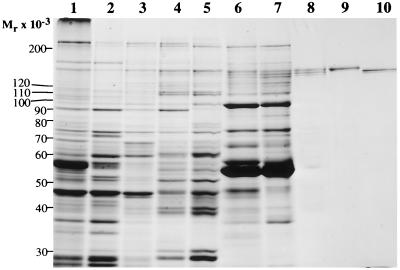
SDS/PAGE of fractions obtained during the purification of IPF. The starting material and fractions containing the peak inhibitory activity from the various purification steps were dissociated by boiling for 2 min in the presence of 1% SDS/5% 2-mercaptoethanol/10% glycerol/63 mM Tris·HCl, pH 6.8 and were subjected to electrophoresis on an SDS/7.5% polyacrylamide gel. Gel staining was with Coomassie brilliant blue. Lanes: 1, 40 μg of calf-brain homogenate; 2, 40 μg of crude cytosol; 3, 40 μg of 45% ammonium sulfate precipitate; 4, 40 μg of PAE 0.5 M NaCl eluate; 5, 40 μg of HAP 0.05 M phosphate eluate; 6, 40 μg of Yellow-86 0.3 M NaCl eluate; 7, 20 μg of gel filtration peak; 8, 5 μg of sucrose gradient peak; 9, 1.5 μg of Mono Q-purified IPF α; 10, 1.5 μg of Mono Q-purified IPF β.
Figure 2.
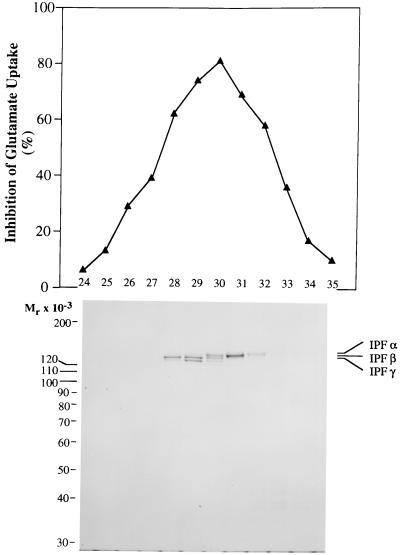
Mono Q HPLC resolution of the IPF αβγ triplet. Peak inhibitory fractions from two 5–20% sucrose density gradients (12 ml, 2 mg of protein) were applied to a Mono Q 5/5 anion-exchange column equilibrated in 75 mM NaCl/1 mM EDTA/0.2 mM PMSF/20 mM Tris·HCl, pH 7.6. Bound protein was eluted in 60 1-ml fractions by a linear NaCl gradient (75–538 mM) developed over 60 min. Aliquots of 10 μl were assayed for inhibitory activity (Upper) and for composition by SDS/PAGE (Lower). All of the inhibitory activity eluted between gradient fractions 24 and 35. Samples for SDS/PAGE were dissociated and electrophoresed as described in the legend for Fig. 1. Gel staining was with Coomassie brilliant blue.
The dye Reactive Yellow-86 was found to have a particularly selective affinity for IPF. The 0.3 M NaCl eluate from this column provided the first glimpse of the IPF αβγ triplet against the protein background (Fig. 1, lane 6). Greater than 90% of the loaded protein did not bind to this column, and although the column effluent contained the majority of the inhibitory activity, no increase in specific activity was achieved.
Sucrose gradient centrifugation proved effective in the purification of IPF because of the anomalous sedimentation behavior of the IPF triplet. IPF α, IPF β, and IPF γ all migrated identically in 5–20% sucrose gradients, with an apparent sedimentation coefficient of 4.3 S. This step usually yielded at least one fraction that was essentially a purified preparation of IPF αβγ.
Fig. 2 shows that high resolution anion-exchange chromatography can partially resolve IPF αβγ into individual components based on assumed differences in net negative charge. It can also be concluded from this figure that both IPF α and IPF β possess inhibitory activity. Fraction 30 (see Fig. 2) usually contained a mixture of IPF α, IPF β, and IPF γ, reminiscent of the starting material, whereas fraction 29 usually contained roughly equal amounts of IPF β and IPF γ. Fraction 28 contained very pure IPF β (135 kDa), and later fractions (31 and 32) contained pure IPF α (138 kDa).
Potency of Purified IPF.
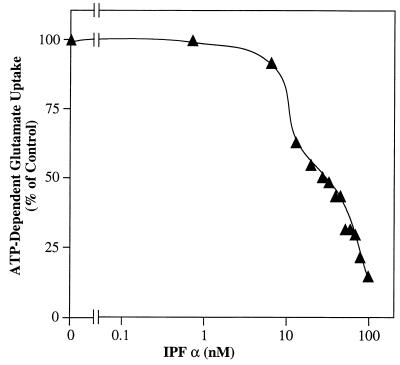
Fig. 3 shows that IPF α is a potent inhibitor of ATP-dependent glutamate uptake in synaptic vesicles. With use of a molecular weight of 138,000 for IPF α, an IC50 of 26 nM was estimated under the standard assay conditions. A similar dose–response curve for IPF β was also generated and indicated an IC50 of 24 nM (data not shown). At approximately 100 nM, both IPF α and IPF β inhibited ATP-dependent glutamate uptake by 90%. However, neither IPF α nor IPF β had any effect on the ATP-independent component of glutamate uptake even at concentrations up to 100 nM (data not shown).
Figure 3.
Dose–response curve for purified IPF α. Purified bovine synaptic vesicles (50 μg of protein) were suspended in the glutamate uptake assay medium in the presence of varying amounts of fraction 31 from the Mono Q column (0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.65, 0.76, 0.86, 0.97, 1.1, 1.3, and 1.6 μg of protein). Using a molecular weight of 138,000 for IPF α, these amounts were converted to nanomoles of IPF α per liter. Uptake was allowed to proceed for 10 min at 30°C, and samples were processed as described in the text. Values for the percentage of control were calculated relative to the ATP-dependent uptake in samples containing equivalent amounts of fraction 31 from a Mono Q gradient run in the absence of loaded protein.
Physicochemical Properties of IPF.
Some of the physicochemical properties are summarized in Table 2. IPF α, IPF β, and IPF γ share highly similar physicochemical properties. The relative molecular weights determined by SDS/PAGE were 138, 135, and 132 × 103 for IPF α, IPF β, and IPF γ, respectively. Their Stokes radii and sedimentation coefficients were indistinguishable from each other, being 60 Å and 4.3 S, respectively. The partial specific volume of IPF α was calculated to be 0.717 ml/g from amino acid composition. Using these values, we calculated an approximate molecular weight of IPF α in the native form to be 103,000, according to the equation M = 6πηNas/(1 - v̄ρ), where n = Avogadro’s number, η = viscosity of water at 20°C, a = Stokes radius, s = sedimentation coefficient at 20°C, v̄ = partial specific volume, and ρ = density of water at 20°C (32). This molecular weight is significantly smaller than that determined by SDS/PAGE. The excessively high Stokes radius and low sedimentation coefficient values for a globular protein indicate that IPF has an elongated shape. This is in agreement with the rather high axial ratio of 12 estimated from the calculated frictional coefficient ratio of 1.67.
Amino Acid Sequence Homology with α Fodrin.
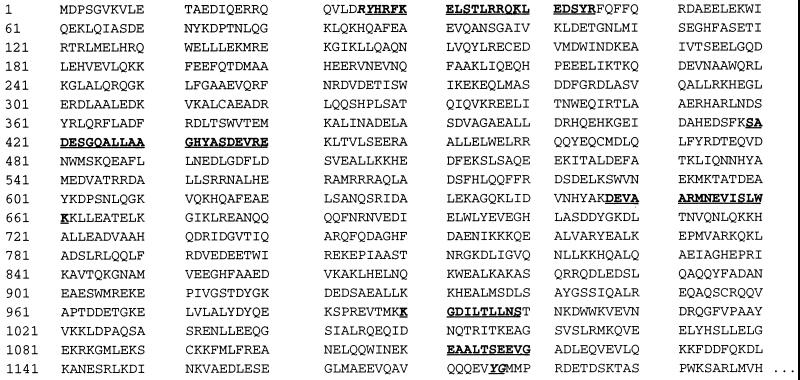
Fig. 4 gives the results of partial sequencing of IPF α. N-terminal sequencing revealed that amino acids 1–20 of IPF α, IPF β, and IPF γ are identical with amino acids 26–45 of human α fodrin (Mr = 240,000). Four further peptides (amino acids 394–415, 622–636, 965–974, and 1086–1095) generated by partial digestion of IPF α confirmed the relationship to α fodrin. Despite this relationship, fodrin purified from whole-brain, according to the method described by Cheney et al. (37), had no effect on glutamate uptake at concentrations up to 1 μM (data not shown).
Figure 4.
Comparison of partial IPF α sequences with the sequence of human α fodrin. Amino acid sequences determined for IPF α are shown in boldface type within the initial 1,200 aa residues of human α fodrin as determined by Moon and McMahon (35). The 20-mer beginning with Tyr-26 represents the N terminus of IPF α. The four internal sequences were determined by sequencing peptides produced by proteolytic digestion of IPF α. The highlighted bond between Tyr-1,176 and Gly-1,177 represents the cleavage site for calpain (36).
Transporter Specificity of Purified IPF α.
To investigate the specificity of the inhibitory effect produced by IPF, its effect on uptake in two other well characterized systems was examined: the Na+-dependent glutamate uptake system in the synaptosomal plasma membrane and the ATP-dependent, reserpine-sensitive catecholamine uptake system in chromaffin vesicles from the adrenal medulla. Results in Fig. 5 indicate that IPF α exhibited no inhibitory effect on Na+-dependent glutamate uptake into bovine synaptosomes. Moreover, IPF α had only a minimal effect (≈18% inhibition) on norepinephrine uptake into bovine chromaffin vesicle ghosts at 100 nM, a concentration that inhibited vesicular glutamate uptake by 90%.
Even though it has not been possible thus far to isolate purified GABAergic vesicles, preliminary experiments with mixed vesicle preparations (containing both glutamate and GABA uptake activities) have shown IPF to be just as potent an inhibitor of vesicular GABA uptake (data not shown).
DISCUSSION
It has been shown previously that glutamate transport into purified synaptic vesicles is inhibited by a soluble factor present in the synaptosomal cytosol (30). We have now purified this factor, referred to as IPF, to apparent homogeneity. The inhibitory activity is associated with three proteins having molecular masses of 138, 135, and 132 kDa (IPF α, IPF β, and IPF γ, respectively). Both IPF α and IPF β share a potent ability to inhibit glutamate uptake into purified synaptic vesicles, and both have a significantly elongated structure. Partial sequence analysis suggests that IPF is derived from α fodrin, a protein implicated in such diverse activities as exocytosis/endocytosis (38), apoptosis (39), and N-methyl-d-aspartate receptor activation (40).
Calculations using the Stokes radius, sedimentation coefficient, and partial specific volume for IPF α indicate a native molecular mass of 103 kDa. This result is at odds with the apparent molecular weight of 138,000 determined by SDS/PAGE. Additionally, the unexpectedly low sedimentation coefficient of 4.3 S is not consistent with the large Stokes radius (60 Å). These data collectively indicate that IPF α is a protein with a markedly elongated structure. This hypothesis is further supported by the large frictional coefficient and axial ratio (see Table 2) determined for IPF α and by the relationship to α fodrin, itself a linear protein. How this rather eccentric structural characteristic might contribute to the ability to inhibit glutamate uptake is not known. The differences in charge between IPF α and IPF β, which render them separable by ion-exchange HPLC (see Fig. 2), apparently have little effect on inhibitory activity, as the two proteins share similar IC50 values.
The precise mechanism by which IPF leads to inhibition of vesicular glutamate uptake remains to be determined. Because transport of glutamate into synaptic vesicles is a coupled process, the possibilities for inhibiting such a system are multiplied by the number of potential coupling sites. Indirect modes of inhibition would include inhibiting the activity of the V-type H+-ATPase, increasing the passive permeability of the vesicle membrane to protons, or causing a generalized increase in membrane permeability (a detergent-like effect). The results in Fig. 5 indicate that these possibilities are unlikely. If IPF were inhibiting the action of the V-type H+-ATPase (either ATP hydrolysis or proton pumping), norepinephrine transport into chromaffin vesicles should also be inhibited. Similarly, any inhibitor that has a generalized protonophore activity would also lead to decreased uptake into chromaffin vesicles. Fig. 5 shows that norepinephrine transport is hardly affected by IPF concentrations up to 100 nM. Finally, the generalized increase in membrane permeability characteristically caused by detergents and other amphiphilic molecules should have an effect on glutamate storage in synaptosomes as well as on norepinephrine uptake. This was not observed.
The relatively high potency of IPF suggests that a more specific process may be involved. The most obvious hypothesis is that IPF causes inhibition of glutamate uptake by a direct interaction with the glutamate translocator protein, either at the glutamate binding site or at an allosteric site. This hypothesis is not supported by the observation that IPF is also a potent inhibitor of GABA uptake in vesicles isolated from rat spinal cord (data not shown). An alternative possibility is that IPF interacts with a synaptic vesicle-specific protein that might lead to a blockade of neurotransmitter storage by an indirect mechanism. Despite this apparent lack of specificity in vitro, specificity in vivo may be enforced by compartmentalization of IPF within particular, neurotransmitter-specific nerve terminals. Regardless of the mechanism of action of IPF, it is a potent, endogenous inhibitor of vesicular neurotransmitter uptake, and therefore of fundamental interest.
The physiological role of IPF remains to be elucidated. The fact that IPF seems to derive from α fodrin is particularly intriguing because fodrin purified from whole brain is itself devoid of inhibitory activity (data not shown). The neutral protease calpain is known to cleave α fodrin at a particular site (see Fig. 4), yielding fragments somewhat larger (150–155 kDa) than those represented by IPF (36, 41). Our results suggest a heretofore undescribed proteolytic action that removes the first 25 aa from α fodrin to yield the N terminus of IPF. This raises the possibility that IPF is derived from α fodrin by at least two proteolytic steps, one generating the N terminus of IPF (Tyr-26) and at least one other (possibly calpain-mediated) yielding the C terminus of IPF, which must occur at or after Gly-1,120 (see Fig. 4). Should this be the case, the resulting conversion of a structural protein to a protein with a presumed regulatory function would be both significant and novel. Alternatively, IPF could be expressed de novo from a distinct gene or possibly by alternative splicing of the full-length α fodrin mRNA before translation.
If IPF indeed acts as an inhibitor of glutamate and/or GABA uptake in vivo, a regulatory mechanism by which IPF can be activated and deactivated must exist. If such a system was shown to exist, it becomes quite conceivable that IPF might play a role in modulating the level of neurotransmitter stored in synaptic vesicles. Such a modulatory activity could have a significant effect on the rapidity of neuronal communication. For example, reducing the glutamate content of a given synaptic vesicle docked at the nerve terminal implies that a smaller amount of this excitatory neurotransmitter would be released into the synaptic cleft per exocytotic event. This could lead to a decrease in response time of the postsynaptic neuron and a resultant lowering of the neuronal firing rate. Conversely, a decrease in GABA content in the synaptic vesicle would lead to facilitation of neuronal firing. Thus, in either case, IPF could play an important role in regulating the speed of synaptic transmission.
Acknowledgments
We are grateful to Drs. Carolyn Bovenkerk and Scott Lewis for assistance in preparing purified synaptic vesicles, and to the laboratory of Dr. David Njus (Department of Biological Sciences, Wayne State University) for providing purified chromaffin vesicles. Dr. Phillip Kish (Department of Neurosurgery, University of Michigan Medical School) kindly provided us with GABA vesicles, purified from rat spinal cord using anti-SV2-conjugated immunobeads. N-terminal sequence analysis was carried out by the University of Michigan Protein and Carbohydrate Structure Facility. Proteolysis and determination of internal sequences were performed by The Rockefeller University Protein/DNA Technology Center. This work was supported by a grant from the National Institutes of Health (Javits Neuroscience Investigator Award NS 26884 to T.U.). F.S.L. was in the Medical Scientist Training Program (GM 07863).
ABBREVIATIONS
- IPF
inhibitory protein factor
- GABA
γ-aminobutyric acid
- PAE
polyethyleneimine
- PMSF
phenylmethylsulfonyl fluoride
- V-type H+-ATPase
vacuolar-type proton translocating ATPase
References
- 1.Watkins J C, Evans R H. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]
- 2.Fonnum F. J Neurochem. 1984;42:1–11. doi: 10.1111/j.1471-4159.1984.tb09689.x. [DOI] [PubMed] [Google Scholar]
- 3.Fagg G E, Foster A C, Ganong A H. Trends Pharmacol Sci. 1986;7:357–363. [Google Scholar]
- 4.Cotman C W, Monaghan D T, Ottersen O P, Storm-Mathisen J. Trends Neurosci. 1987;10:273–279. [Google Scholar]
- 5.Cotman C W, Bridges R J, Taube J S, Clark A S, Geddes J W, Monaghan D T. J NIH Res. 1989;1:65–74. [Google Scholar]
- 6.Nakanishi S. Science. 1992;258:597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- 7.Greenamyre J T. Arch Neurol. 1986;43:1058–1063. doi: 10.1001/archneur.1986.00520100062016. [DOI] [PubMed] [Google Scholar]
- 8.Rothman S M, Olney J W. Trends Neurosci. 1987;10:299–302. [Google Scholar]
- 9.Meldrum B. Epilepsia. 1991;32:S1–S3. doi: 10.1111/j.1528-1157.1991.tb05879.x. [DOI] [PubMed] [Google Scholar]
- 10.Coyle J T, Puttfarcken P. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 11.Williamson P C. Arch Neurol. 1993;50:1096–1097. doi: 10.1001/archneur.1993.00540100081021. [DOI] [PubMed] [Google Scholar]
- 12.Collingridge G L, Bliss T V P. Trends Neurosci. 1987;10:288–293. [PubMed] [Google Scholar]
- 13.Zheng F, Gallagher J P. Neuron. 1992;9:163–172. doi: 10.1016/0896-6273(92)90231-2. [DOI] [PubMed] [Google Scholar]
- 14.Bashir Z I, Bortolotto Z A, Davies C H, Berretta N, Irving A J, Seal A J, Henley J M, Jane D E, Watkins J C, Collingridge G L. Nature (London) 1993;363:347–350. doi: 10.1038/363347a0. [DOI] [PubMed] [Google Scholar]
- 15.Naito S, Ueda T. J Biol Chem. 1983;258:696–699. [PubMed] [Google Scholar]
- 16.Naito S, Ueda T. J Neurochem. 1985;44:99–109. doi: 10.1111/j.1471-4159.1985.tb07118.x. [DOI] [PubMed] [Google Scholar]
- 17.Maycox P R, Deckwerth T, Hell J W, Jahn R. J Biol Chem. 1988;263:15423–15428. [PubMed] [Google Scholar]
- 18.Cidon S, Sihra T S. J Biol Chem. 1989;264:8281–8288. [PubMed] [Google Scholar]
- 19.Fykse E M, Christensen H, Fonnum F. J Neurochem. 1989;52:946–951. doi: 10.1111/j.1471-4159.1989.tb02546.x. [DOI] [PubMed] [Google Scholar]
- 20.Tabb J S, Ueda T. J Neurosci. 1991;11:1822–1828. doi: 10.1523/JNEUROSCI.11-06-01822.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Storm-Mathisen J, Leknes A K, Bore A, Vaaland J L, Edminson P, Haug F M S, Ottersen O P. Nature (London) 1983;301:517–520. doi: 10.1038/301517a0. [DOI] [PubMed] [Google Scholar]
- 22.Burger P M, Mehl E, Cameron P L, Maycox P R, Baumert M, Lottspeich F, DeCamilli P, Jahn R. Neuron. 1989;3:715–720. doi: 10.1016/0896-6273(89)90240-7. [DOI] [PubMed] [Google Scholar]
- 23.Nicholls D G, Sihra T. Nature (London) 1986;321:772–773. doi: 10.1038/321772a0. [DOI] [PubMed] [Google Scholar]
- 24.McMahon H T, Nicholls D G. Biochim Biophys Acta. 1991;1059:243–264. doi: 10.1016/s0005-2728(05)80210-5. [DOI] [PubMed] [Google Scholar]
- 25.Kish P E, Ueda T. Neurosci Lett. 1991;122:179–182. doi: 10.1016/0304-3940(91)90852-k. [DOI] [PubMed] [Google Scholar]
- 26.Mehta P P, Battenberg E, Wilson M E. Proc Natl Acad Sci USA. 1996;93:10471–10476. doi: 10.1073/pnas.93.19.10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tabb J S, Kish P E, Van Dyke R, Ueda T. J Biol Chem. 1992;267:15412–15418. [PubMed] [Google Scholar]
- 28.Shioi J, Naito S, Ueda T. Biochem J. 1989;258:499–504. doi: 10.1042/bj2580499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ueda T. In: Excitatory Amino Acids. Roberts P J, Storm-Mathisen J, Bradford H F, editors. London: Macmillan; 1986. pp. 173–195. [Google Scholar]
- 30.Lobur A T, Kish P E, Ueda T. J Neurochem. 1990;54:1614–1618. doi: 10.1111/j.1471-4159.1990.tb01212.x. [DOI] [PubMed] [Google Scholar]
- 31.Kish P E, Ueda T. Methods Enzymol. 1989;174:9–25. doi: 10.1016/0076-6879(89)74005-2. [DOI] [PubMed] [Google Scholar]
- 32.Siegel L M, Monty K J. Biochim Biophys Acta. 1966;112:346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- 33.Martin R G, Ames B N. J Biol Chem. 1960;236:1372–1379. [PubMed] [Google Scholar]
- 34.Tanford C. Physical Chemistry of Macromolecules. New York: Wiley; 1961. [Google Scholar]
- 35.Moon R T, McMahon A P. J Biol Chem. 1990;265:4427–4433. [PubMed] [Google Scholar]
- 36.Harris A S, Croall D E, Morrow J S. J Biol Chem. 1988;263:15754–15761. [PubMed] [Google Scholar]
- 37.Cheney R, Levine J, Willard M. Methods Enzymol. 1986;134:42–54. doi: 10.1016/0076-6879(86)34074-6. [DOI] [PubMed] [Google Scholar]
- 38.Goodman S R, Zimmer W E, Clark M B, Zagon I S, Barker J E, Bloom M L. Brain Res Bull. 1995;36:593–606. doi: 10.1016/0361-9230(94)00264-2. [DOI] [PubMed] [Google Scholar]
- 39.Martin S J, O’Brien G A, Nishioka W K, McGahon A J, Mahboubi A, Saido T C, Green D R. J Biol Chem. 1995;270:6425–6428. doi: 10.1074/jbc.270.12.6425. [DOI] [PubMed] [Google Scholar]
- 40.Siman R, Baudry M, Lynch G. Nature (London) 1985;313:225–228. doi: 10.1038/313225a0. [DOI] [PubMed] [Google Scholar]
- 41.Seubert P, Lee K, Lynch G. Brain Res. 1989;492:366–370. doi: 10.1016/0006-8993(89)90921-9. [DOI] [PubMed] [Google Scholar]