Abstract
γ-Aminobutyric acid type A receptors (GABAA-Rs) mediate the bulk of rapid inhibitory synaptic transmission in the central nervous system. The β3 subunit is an essential component of the GABAA-R in many brain regions, especially during development, and is implicated in several pathophysiologic processes. We examined mice harboring a β3 gene inactivated by gene targeting. GABAA-R density is approximately halved in brain of β3-deficient mice, and GABAA-R function is severely impaired. Most β3-deficient mice die as neonates; some neonatal mortality, but not all, is accompanied by cleft palate. β3-deficient mice that survive are runted until weaning but achieve normal body size by adulthood, although with reduced life span. These mice are fertile but mothers fail to nurture offspring. Brain morphology is grossly normal, but a number of behaviors are abnormal, consistent with the widespread location of the β3 subunit. The mice are very hyperactive and hyperresponsive to human contact and other sensory stimuli, and often run continuously in tight circles. When held by the tail, they hold all paws in like a ball, which is frequently a sign of neurological impairment. They have difficulty swimming, walking on grids, and fall off platforms and rotarods, although they do not have a jerky gait. β3-deficient mice display frequent myoclonus and occasional epileptic seizures, documented by electroencephalographic recording. Hyperactivity, lack of coordination, and seizures are consistent with reduced presynaptic inhibition in spinal cord and impaired inhibition in higher cortical centers and/or pleiotropic developmental defects.
Keywords: gene targeting, benzodiazepine, Angelman syndrome, anesthesia
γ-Aminobutyric acid type A receptors (GABAA-Rs) mediate the bulk of rapid inhibitory synaptic transmission in the central nervous system. GABAA-Rs are heteromeric ligand-gated chloride channels that are encoded by at least 15 separate subunit genes and they form a number of binding sites for clinically important drugs such as benzodiazepines, barbiturates, and anesthetics (1, 2). The β3 subunit is a major constituent of the GABAA-R in selected regions, especially cerebral cortex, hippocampal formation, hypothalamus, cranial nerve ganglia, and spinal cord in adults, and even more widespread and abundant in prenatal and neonatal brain, suggesting an important developmental role (3, 4). The β3 gene is clustered with two other GABAA-R subunits (α5 and γ3) on mouse chromosome 7; deletion of these three genes and surrounding loci results in neonatal mortality, craniofacial abnormalities, and neurological signs (5–7). The syntenic region on human chromosome 15, including the cluster of three GABAA-R subunits, is the area associated with the human genetic disease Angelman syndrome (8–10). The gene for the GABAA-R β3 subunit has been proposed as a candidate for some of the phenotypic features of this syndrome (8, 11). To test directly the physiologic and developmental functions of the β3 subunit, we examined mice harboring a β3 gene inactivated by gene targeting.
METHODS
Production of Knockout Mice.
Strain 129 mouse genomic DNA was isolated from a P1 phage library (Genome Systems, St. Louis). Details of library screening and vector construction are available upon request. Following electroporation of R1 (12) embryonic stem (ES) cells, clones were screened for targeting by Southern blot analysis of EcoRV-digested genomic DNA. Blots were hybridized with a 3′ probe that is external to the targeting construct. This probe is a 1.1-kb BglII–BamHI genomic subclone. Targeting was confirmed with several other restriction digests and by hybridization with a neomycin phosphotransferase probe and with a 5′ arm probe (data not shown). Two correctly targeted ES cell clones (β3#347, β3#372) were transferred through the germ line. Results presented here are from the β3#347 clone. A cursory analysis of β3#372 yielded similar results. The genetic background of all mice was Strain 129 × C57BL/6J F2 or F3. These mice have been given the strain designation Gabrb3tm1Geh and are available from the Induced Mutant Resource (The Jackson Laboratories). For Northern blot analysis, poly(A)+ RNA was isolated from whole brain of neonatal mice using the Microfast Track kit (Invitrogen). The β3 exon 1–2 probe is a genomic DNA restriction fragment. The β3 exon 9 probe is a PCR product (10). Human β-actin (CLONTECH) was used as a probe to assess the integrity of the RNA and as a control for the amount of RNA loaded.
Analysis of Palate Morphology.
Neonate heads were collected and fixed on the day of birth. We detected a slight difference in the overall head sizes, with the β3−/− heads smaller than pcp/pcp heads. This difference is likely due to strain variation as β3+/− and pcp/p6H show a similar size difference. To minimize the measurements being biased by the size of individual heads, the ratio, W½/L (width of cleft at the midsection divided by anterior-posterior length of cleft), was calculated.
GABAA-R Pharmacology.
Binding of a prototypic GABA site agonist ([3H]muscimol) and an imidazobenzodiazepine ([3H]Ro15-4513) that recognizes all known central benzodiazepine sites (at six different concentrations, each in quadruplicate) to whole brain homogenates was measured using a centrifugation method (13, 14). Data were pooled from three to four separate experiments using two separate membrane preparations (≈40 neonatal brains and four adult brains per membrane preparation, respectively) and fit to a logistic function using an iterative nonlinear least squares method, yielding binding parameters and their standard errors. Parameters were compared using the z statistic (15).
For autoradiography, horizontal and sagittal serial sections (20 μm) from postnatal day 1 mice (n = 6 for each genotype) were preincubated for 15 min at 0–4°C in 0.31 M Tris-citrate (pH 7.1) or 50 mM Tris·HCl (pH 7.4) supplemented with 120 mM NaCl for the GABA and benzodiazepine sites, respectively. The GABA sites were labeled by [3H]muscimol (20 nM; Amersham) (16) during a 30-min incubation at 0–4°C. The benzodiazepine sites were labeled by [3H]Ro15-4513 (10 nM; DuPont/NEN) (17) with and without zolpidem (Synthelabo Recherche, Bagneux, France) during a 60-min incubation at 0–4°C. The incubations were carried out in the respective preincubation buffers, and terminated by three washes, a dip in distilled water, and quick air-drying under a fan. The sections were apposed to Hyperfilm-3H (Amersham) for 6–12 weeks. Nonspecific binding to the GABA and benzodiazepine sites was determined in the presence of 100 μM GABA (Sigma) or 10 μM flumazenil (Ro15-1788; Hoffmann–La Roche), respectively. Labeling densities of several brain regions was quantitated from the films by using MCID M4 image analysis system (Imaging Research, St. Catharines, Ontario, Canada), with the reference to simultaneously exposed 3H-plastic standards (Amersham) (18).
Electrophysiology.
Dorsal root ganglion neurons (DRGs) were dissociated from postnatal day 1 mouse pups as described by White (19), plated onto poly-d-lysine coated coverslips and used for recordings within 12 h. Coverslips containing neurons were transferred to a chamber which was continuously perfused with extracellular medium containing 145 mM NaCl, 3 mM KCl, 1.5 mM CaCl2, 1 mM MgCl2, 6 mM d-glucose, and 10 mM Hepes/NaOH (pH 7.4). Recordings were made using the whole-cell patch-clamp technique. Patch pipettes contained 145 mM N-methyl d-glucamine hydrochloride, 5 mM K2ATP, 1.1 mM EGTA, 2 mM MgCl2, 5 mM Hepes/KOH (pH 7.2), and 0.1 mM CaCl2; pipette resistance was 4–5 MΩ. Neurons were voltage clamped at −60 mV. GABA was rapidly applied (>1 ml/min) to the cell by local perfusion (20) using a motor-driven solution exchange device (Rapid Solution Changer 100; Molecular Kinetics, Pullman, WA). Responses were digitized (TL-1–125 interface; Axon Instruments, Foster City, CA) using pclamp, version 5 (Axon Instruments). Numerical data are presented throughout as mean ± SEM.
Electroencephalogram (EEG) Analysis.
Seven mice of each genotype, 2–6 months of age, had EEG recordings performed via epidural screw electrodes implanted above right and left parietal cortex, and a reference electrode in the nasal bone.
RESULTS AND DISCUSSION
Production and Molecular Characterization of Mice.
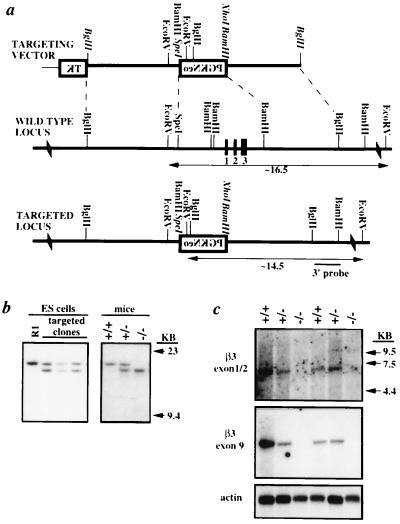
We used homologous recombination in mouse ES cells to disrupt the β3 gene (see Fig. 1 a and b). Of 71 ES cell clones analyzed, 4 displayed predicted restriction fragment lengths indicative of correct gene targeting. The 3′ probe hybridized only to a ≈16.5-kb EcoRV fragment from the wild-type allele in the parental ES cell line (R1) and wild-type mice. This probe also hybridized to an ≈14.5-kb fragment from a correctly targeted allele. Following transmission of the targeted β3 gene through the mouse germ line, heterozygous (β3+/−) mice were intercrossed to produce wild-type (β3+/+), β3+/−, and homozygous (β3−/−) mice for analysis.
Figure 1.
Targeted disruption of the β3 locus. (a) A replacement vector was constructed to replace ≈2.8 kb of β3 genomic DNA including ≈1.8 kb of promoter and exons 1–3 with the selectable marker neomycin phosphotransferase (PGKNeo). (Note that restriction sites set in italics were destroyed during vector construction.) (b) Southern blot analyses of the parental cell line (R1), three correctly targeted ES cell clones, and mice demonstrating all genotypes. (c) Northern blot analysis of neonatal mouse brain poly(A)+ RNA that demonstrates the absence of β3 mRNA in brain of β3−/− mice.
The modified β3 gene was verified to be nonfunctional (i.e., a null allele) by Northern blot analysis. Hybridization of neonatal whole brain poly(A)+ RNA with probes specific for exons 1–2 and exon 9 revealed the complete absence of the ≈6.0-kb β3 mRNA in β3−/− mice (Fig. 1c). Densitometric analysis of autoradiographs indicated an ≈30% reduction in β3 mRNA in β3+/− mice.
Neonatal Mortality and Cleft Palate.
Approximately 90% of β3−/− mice die within 24 h of birth. This frequency is similar to that observed in two known radiation-induced pink-eyed dilution (p) mutants (pcp and p4THO-II) that lack the β3, α5, and γ3 subunits of the GABAA-R (5, 21, 22). The cause of death in these p locus mutants has been attributed to feeding problems that result from cleft palate. This phenotype can be rescued by transgenic reintroduction of the β3 subunit cDNA in some homozygous p4THO-II mice (6). Interestingly, in the β3−/− mice described here, penetrance of cleft palate was only ≈57% (40/70), markedly lower than the 95% observed in the radiation-induced mutants. This may reflect differences in genetic background or the contribution of other genes deleted in the radiation-induced mutants.
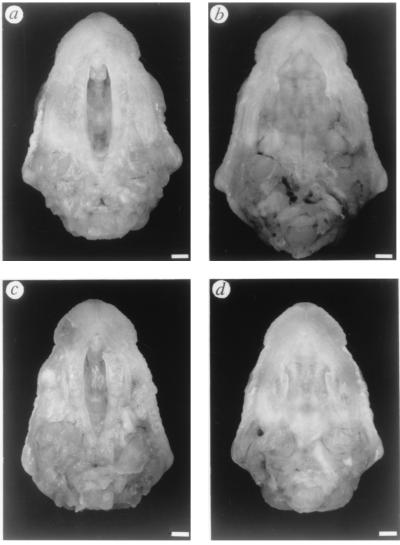
We also compared the size of the cleft between β3−/− and pcp homozygotes and surprisingly found no significant difference between these two mutants (Fig. 2). Mean and SEM of ratios obtained for β3−/− and pcp/pcp were 0.23 ± 0.01 (n = 10) and 0.24 ± 0.01 (n = 10), respectively. The ranges of direct measurements of each group are L = 4.5–5.7 mm, W½ = 0.89–1.6 mm (β3−/−) and L = 4.4–6.0 mm, W½ = 0.86–1.6 mm (pcp/pcp). The observation that the lower penetrance of cleft palate has little effect on severity of the palate lesion may suggest that induction of cleft palate is regulated by threshold mechanisms. Reaching the threshold to induce cleft palate may be fulfilled by multiple factors, whose effects are augmented by the absence of β3 gene product, somehow more strongly in pcp and p4THO-II, possibly due to the additional loss of the α5 and γ3 subunits. This multiple factor threshold hypothesis is appealing, since toxicological studies of cleft palate induction suggest that there are multiple pathways involved in the formation of cleft palate (see ref. 23–26). As noted by Culiat et al. (6, 22), paradoxically both the loss of a GABAA-R as well as the addition of GABA (27) or the GABA agonist, diazapam (28), all promote cleft palate. Taken together these studies indicate the importance of the GABAergic system in normal palate development.
Figure 2.
Palate morphology of pcp and targeted β3 neonates. (a) pcp/pcp. (b) pcp/p6H. (c) β3−/−. (d) β3+/−. The pcp/p6H and the β3+/− mice each have one wild-type β3 gene and do not exhibit cleft palate. (Bar = 1 mm.)
We do not know at this time why ≈30% of β3−/− mice with apparently normal palates die neonatally. The fact that these mice do die in the same time frame as those with palate defects calls into question the assumption that it is the clefting defect alone that results in the lethal phenotype. The cause of death cannot be attributed to defects in gross morphology, as the brains of these mice are morphologically normal (data not shown). This confirms the observation reported for pcp homozygous brain (5).
GABAA-R Pharmacology.
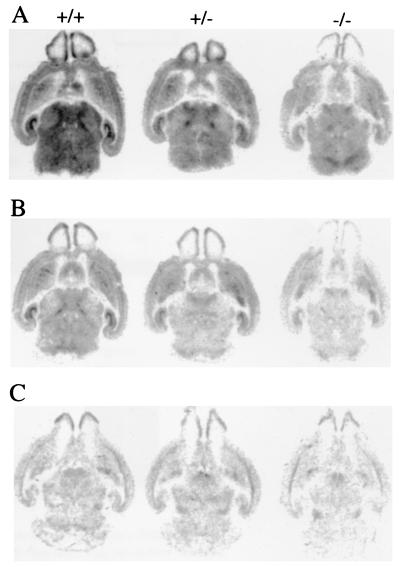
The contribution that β3 subunits make to the population of heteropentameric GABAA-Rs was studied by comparing binding of [3H]muscimol and [3H]Ro15-4513 as ligands to label GABA and benzodiazepine sites, respectively. GABAA-R density is remarkably reduced in β3−/− mice. Binding of [3H]muscimol and [3H]Ro15-4513 was approximately halved in brain homogenates of β3−/− mice compared with β3+/+, without any change in affinity (Table 1). Autoradiographic analysis of newborn mouse brains also indicated that β3−/− mice have lower binding for both ligands (Fig. 3 A and C). [3H]Ro15-4513 labeled less sites in β3−/− mice than in β3+/+ or β3+/− mice, the labeling being significantly lower in all regions quantitated (Table 2). A saturating concentration (30 μM) of zolpidem, a GABAA-R isoform selective benzodiazepine site agonist that is unable to bind to α5 and/or γ3 subunit-containing receptors (29, 30), displaced similar proportions of benzodiazepine binding in all three genotypes (Fig. 3B). This indicates that some zolpidem-insensitive receptors, presumably containing α5 and γ3, persist in the β3−/− mice. The more quantitative measurements on total brain homogenates showed a significant reduction in the IC50 (increased potency) for zolpidem displacement of benzodiazepine in β3−/− mice (Table 1), indicating that some α5 and γ3 subunits depend on the presence of β3 for incorporation into GABAA-R heteropentameric isoforms.
Table 1.
Binding of [3H]muscimol and [3H]Ro15-4513 to whole brain homogenates prepared from postnatal day 1 (pups) or 8- to 12-week-old (adult) mice
| Binding parameter | Wild type (β3+/+) | Homozygous (β3−/−) | P |
|---|---|---|---|
| [3H]Muscimol (pups) | |||
| Bmax, pmol/mg | 3.0 ± 0.2 | 1.5 ± 0.2 | <0.001 |
| Kd, nM | 10.8 ± 1.1 | 9.1 ± 3.3 | 0.62 |
| [3H]Ro15-4513 (pups) | |||
| Bmax, pmol/mg | 1.5 ± 0.1 | 0.7 ± 0.1 | <0.001 |
| Kd, nM | 8.0 ± 2.9 | 8.8 ± 3.4 | 0.86 |
| [3H]Ro15-4513, adult | |||
| Bmax, pmol/mg | 3.1 ± 0.3 | 1.7 ± 0.1 | 0.01 |
| Zolpidem + [3H]Ro15-4513 (pups) IC50, μM | 0.54 ± 0.09 | 0.15 ± 0.04 | <0.001 |
Figure 3.
Autoradiographic binding of a GABA site ligand ([3H]muscimol) and a benzodiazepine site ligand ([3H]Ro15-4513) to representative horizontal brain sections from newborn wild-type (+/+), heterozygous (+/−), and homozygous (−/−) mice. (A) Total binding of 5 nM [3H]Ro15-4513. (B) Binding of [3H]Ro15-4513 in the presence of 30 μM zolpidem. (C) Total binding of 20 nM [3H]muscimol. All images represent specific binding since [3H]Ro15-4513 and [3H]muscimol failed to label any sites in the presence of 10 μM flumazenil and 100 μM GABA, respectively.
Table 2.
Binding of [3H]muscimol and [3H]Ro15-4513 to postnatal day 1 mouse brain sections as revealed by autoradiography
| Brain region | [3H]Muscimol, nCi/mg | [3H]Ro15-4513, nCi/mg |
|---|---|---|
| Frontoparietal cortex | ||
| β3+/+ | 0.80 ± 0.14 | 3.70 ± 0.84 |
| β3+/− | 0.72 ± 0.10 | 2.58 ± 0.33 |
| β3−/− | 0.58 ± 0.09* | 2.59 ± 0.52* |
| Olfactory bulbs | ||
| β3+/+ | 0.65 ± 0.08 | 2.43 ± 0.40 |
| β3+/− | 0.55 ± 0.07 | 2.16 ± 0.46 |
| β3−/− | 0.59 ± 0.05 | 1.00 ± 0.08† |
| Caudate/putamen | ||
| β3+/+ | 0.66 ± 0.07 | 3.42 ± 0.63 |
| β3+/− | 0.64 ± 0.14 | 3.15 ± 0.34 |
| β3−/− | 0.53 ± 0.10 | 2.57 ± 0.37* |
| Thalamus | ||
| β3+/+ | 0.83 ± 0.16 | 4.92 ± 0.62 |
| β3+/− | 0.81 ± 0.22 | 3.79 ± 0.40 |
| β3−/− | 0.52 ± 0.06* | 2.51 ± 0.85† |
| Hippocampus | ||
| β3+/+ | 0.70 ± 0.11 | 3.68 ± 0.90 |
| β3+/− | 0.64 ± 0.14 | 2.96 ± 0.80 |
| β3−/− | 0.57 ± 0.16 | 2.22 ± 0.48* |
Optical density values in relation to radioactivity standards are means ± SEM for three to six brains. THIP (4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol) (10 μM) was able to reduce [3H]muscimol binding to the background level (data not shown). The statistical significance of differences between β3+/+, β3+/−, and β3−/− mice was determined using one-way ANOVA followed by Bonferroni post test (∗, P < 0.05;
, P < 0.001).
The binding of [3H]muscimol assayed by autoradiography also was decreased in β3−/− mice, but significant decreases were observed only in the cortex and thalamus. [3H]Muscimol binding may, however, detect only high affinity subpopulations of GABAA-Rs (16), with presently unknown subunit composition. Because benzodiazepine binding is decreased more widely than [3H]muscimol binding, the latter may emphasize GABAA-R isoforms that do not contain β3, as suggested by studies on pcp mice (8).
The autoradiographic data indicate that the β3 subunit is widely expressed in the mouse brain, possibly assembling with several α and γ subunit variants. The reduced binding observed for the GABA and benzodiazepine ligands demonstrates that another subunit such as β2 is unable to substitute for the β3 subunit. This results in a marked deficit in GABAA-R level in the β3−/− animals. These observations are consistent with largely separate receptor populations containing β2 and β3 subunits in the rat brain (31).
Electrophysiology.
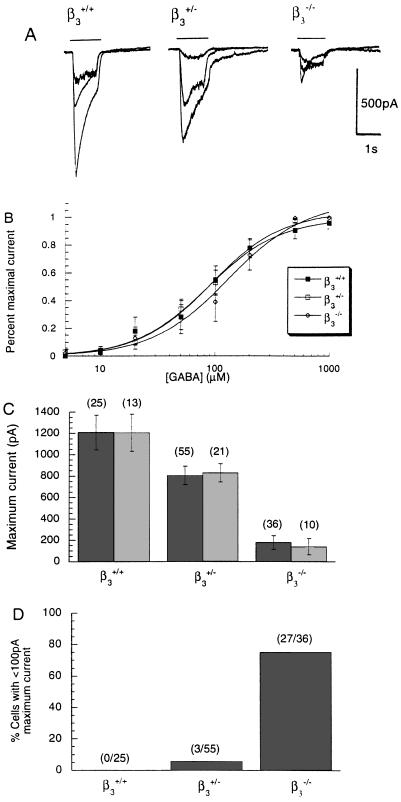
In situ hybridization studies of neonatal rat have demonstrated that sensory neurons of the DRG express high levels of β3 mRNA (32). Electrophysiologic recordings from sensory neurons isolated from the DRG of neonatal mice showed a dramatic (≈80%) decrease in the maximal amplitude of GABA-activated chloride currents in a population of neurons from β3−/− mice relative to cells taken from β3+/+ siblings (P < 0.001, Student’s t test; Fig. 4 A and C). In addition, there was a significant decrease (25%) in the GABA current amplitude in neurons from β3+/− mice relative to β3+/+ mice (P < 0.05, Student’s t test; Fig. 4C). A large fraction (27/36) of the neurons tested from β3−/− animals responded to GABA with <100 pA of current, corresponding to <10% of the mean from control neurons (Fig. 4D). No neurons from β3+/+ animals fell into this range. Interestingly, however, a few neurons from β3−/− animals gave large currents. The concentration-response curve for GABA was not significantly different in neurons from the β3+/− or β3−/− mice (Fig. 4B).
Figure 4.
GABA-evoked whole cell currents from acutely dissociated DRG neurons of newborn β3+/+, β3+/−, and β3−/− mice. (A) Representative currents from individual neurons from each group in response to GABA (10 μM, 100 μM, and 1 mM). The bar above the trace represents duration of GABA application. (B) Pooled GABA concentration-response curves (4 < n < 7), yielding EC50 values of 86.1 ± 9.3 μM (+/+), 92.1 ± 6.0 μM (+/−), and 131.0 ± 23.0 μM (−/−). Hill coefficients were 1.4, 1.4, and 1.3. (C) Pooled data for maximal GABA current amplitudes (mean ± SEM) expressed for all neurons tested (dark shading) and for the average current from neurons in each mouse (light shading). (D) The majority of neurons from β3−/− mice produced <100 pA maximal current. In contrast, only a small minority of neurons from β3+/− mice and none from β3+/+ animals produced such small currents in response to GABA.
The results from isolated DRG neurons suggest that the majority of the GABAA-Rs on the cell bodies of these sensory neurons in the neonatal mouse are β3-containing, consistent with in situ hybridization studies in the neonatal rat (32). The small GABA-evoked currents in neurons from β3−/− animals were sensitive to potentiation by the anticonvulsant loreclezole (5 μM; n = 4; data not shown), consistent with the remaining functional GABAA-Rs being β2-containing rather than β1-containing (33). Since GABAA-Rs are found on the terminals of sensory afferents in the dorsal and ventral horn of the spinal cord, where they are responsible for presynaptic inhibition (34), we suggest that some of the motor manifestations of hyperexcitability in the β3-deficient animals result from a decrease in the effectiveness of spinal presynaptic inhibition. This in turn is due to the decline in numbers of GABAA-Rs on the terminals of primary afferents, consistent with the loss of β3-containing receptors observed at the cell body level in our experiments in the β3−/− animals. The smaller but statistically highly significant receptor deficit seen in the heterozygotes suggests the possibility of reduced spinal presynaptic inhibition in these animals also.
Additional Abnormalities.
The ≈5–10% of β3−/− mice that survive to adulthood display several behavioral and physiological abnormalities. The extent of these abnormalities varies greatly from mouse to mouse. Most β3−/− mice are runted until weaning but they achieve normal body weight by adulthood. The mice are very hyperactive and hyperresponsive to human contact and other sensory stimuli. Many, but not all, β3−/− mice have been observed to run continuously in tight circles for extended periods of time. When held by the tail, they hold all paws in like a ball, which is frequently a sign of neurological impairment. They have difficulty swimming, walking on grids, and repeatedly fall off platforms and rotarods. In marked contrast to the exceptional surviving p locus mutant mice mentioned above, which are also runted and nervous (5, 21, 22), β3−/− mice do not display jerky gait. Fertility is normal but β3−/− mothers fail to display appropriate nurturing behavior irrespective of the genotype of the offspring. Longevity of β3−/− mice that survive the neonatal period is reduced to an average age of 18 weeks (range, 9–41 weeks, n = 20) in contrast to an expected 127 weeks; the cause of premature death is unknown.
Seizures and EEG Analysis.
Male and female β3−/− mice were observed up to ≈10 months of age. They displayed recurring hyperactive behavior that was interrupted by periods of arrest lasting seconds to minutes. Myoclonic body jerks and epileptic seizures were observed in mice over 10 weeks of age, especially in older mice. Seizures varied in severity; the mildest seizures consisted of twitching of muscles of the mouth, face, whiskers, and ears. More severe seizures included head and bilateral forelimb jerks, with or without opisthotonos, arching of the tail, and falls. In the most severe seizures, convulsions were succeeded by a wild running/bouncing phase. Seizures were noted clinically in 10 of 24 mice by chance observation. Closer observation during EEG analysis revealed seizures in 4 of 4 β3−/− mice. Thus, seizures are likely present in all β3−/− mice.
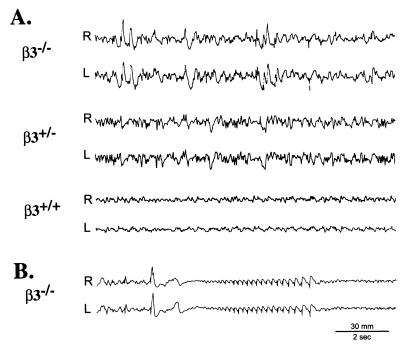
EEGs of β3−/− mice had relatively normal background, especially during maximal alertness, but were frequently interrupted by higher amplitude polymorphic slow and sharp waves, corresponding to behavioral quiescence (Fig. 5A). This abnormal EEG pattern stopped on arousal. High-amplitude generalized interictal spikes were frequently observed. These abnormal epileptiform patterns were seen in 7 of 7 β3−/− animals examined. Seizures were associated with generalized spike/waves. A large spike frequently appeared immediately prior to or at the start of ictus and synchronized buildup, followed by postictal depression (Fig. 5B). β3+/+ mice did not show these abnormal EEG patterns, whereas β3+/− mice exhibited patterns intermediate between β3−/− and β3+/+ mice (Fig. 5A). Lack of seizures in wild-type mice is consistent with the well-known seizure resistance of C57BL/6J mice (35). As an additional control experiment, two Strain 129/SvJ mice (age 15 weeks) were recorded for 3 h on two different days; no EEG abnormalities occurred.
Figure 5.
Electroencephalographic recordings. (A) Three animals (β3−/−, β3+/−, β3+/+) were simultaneously recorded at 2 months of age. The β3−/− record (Top) is abnormal, displaying abundant paroxysmal slow and sharp wave activity, in marked contrast to the normal pattern of β3+/+ littermate controls (Bottom). β3+/− mice display a pattern intermediate between that of β3−/− and β3+/+ records. (B) Seizure in β3−/− mouse at 5 months of age, followed by suppression of electrocortical activity.
CONCLUSION
To our knowledge, no previous study has established that alterations in GABAA-Rs underlie any type of animal or human epilepsy. The results presented here provide evidence that a reduction in certain GABAA-R subunit proteins, in this case the β3 subunit, can cause epilepsy. The human genetic disorder Angelman syndrome is characterized by severe mental retardation and epilepsy (36–38). This syndrome is produced by deletion or mutation in maternal chromosome 15q11–13 (39), an area to which the genes for the α5, γ3, and β3 subunits of the GABAA-R map (9). Studies in progress are comparing the features of the seizures in β3−/− knockout mice and other abnormal behaviors with those seen in human diseases, including Angelman syndrome.
Selective knockout of the β3 subunit of the GABAA-R also produces the cleft palate and neurological abnormalities seen in large genetic deletions that encompass numerous genes including a cluster of three GABAA-R subunits. The phenotypic characteristics of β3−/− mice are consistent with the expected results of a deficiency of GABA-mediated inhibition in adult brain and spinal cord and possibly a deficiency in developmental functions subserved by GABA. The suspected links between GABA and epilepsy and GABA and brain development are now more firmly established.
Acknowledgments
We thank Jodi Daggett, Frank Kist, Anton Asatourian, and Joanne Steinmiller for their expert technical assistance, and Janssen Pharmaceutical for loreclezole. We also thank Drs. G. White, D. McGehee, and K. Houamed for their advice on DRG dissection and dissociation; Dr. A. Klein-Szanto for brain histology and neonate dissection; and Drs. A. V. Delgado-Escueta and B. Minassian for discussion of seizures and EEGs. This work was supported by the University of Pittsburgh Anesthesiology/Critical Care Medicine Foundation, the Academy of Finland (Grant 37453 to E.R.K.), and by the National Institutes of Health (Grant AA10422 to G.E.H., Grant GM52035 to L.L.F., and Grant GM43840 to M.H.B.).
ABBREVIATIONS
- GABA
γ-aminobutyric acid
- GABAA-R
GABA type A receptor
- ES
embryonic stem cell
- DRG
dorsal root ganglion
- EEG
electroencephalogram
References
- 1.Stephenson F A. Biochem J. 1995;310:1–9. doi: 10.1042/bj3100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKernan R, Whiting P. Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- 3.Laurie B J, Wisden W, Seeburg P H. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wisden W, Laurie D J, Monyer H, Seeburg P H. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakatsu Y, Tyndale R F, DeLorey T M, Durham-Pierre D, Gardner J M, McDanel H J, Nguyen Q, Wagstaff J, Lalande M, Sikela J M, Olsen R W, Tobin A J, Brilliant M H. Nature (London) 1993;364:448–450. doi: 10.1038/364448a0. [DOI] [PubMed] [Google Scholar]
- 6.Culiat C T, Stubbs L J, Woychik R P, Russell L B, Johnson D K, Rinchik E M. Nat Genet. 1995;11:344–346. doi: 10.1038/ng1195-344. [DOI] [PubMed] [Google Scholar]
- 7.Culiat C T, Stubbs L J, Montgomery C S, Russell L B. Proc Natl Acad Sci USA. 1994;91:2815–2818. doi: 10.1073/pnas.91.7.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeLorey T, Minassian B, Tyndale R, Olsen R. In: GABA Receptors, Transporters, and Metabolism. Tanaka C, Bowery N, editors. Basel: Birkhauser; 1996. pp. 275–282. [Google Scholar]
- 9.Greger V, Knoll J H, Woolf E, Glatt K, Tyndale R F, DeLorey T M, Olsen R W, Tobin A J, Sikela J M, Nakatsu Y, Brilliant M H, Whiting P J, Lalande M. Genomics. 1995;26:258–264. doi: 10.1016/0888-7543(95)80209-5. [DOI] [PubMed] [Google Scholar]
- 10.Wagstaff J, Knoll J H M, Fleming J, Kirkness E F, Martin-Gallardo A, Greenberg F, J M, Grahm J, Menninger J, Ward D, Venter J C, Lalande M. Am J Hum Genet. 1991;49:330–337. [PMC free article] [PubMed] [Google Scholar]
- 11.Minassian, B., DeLorey, T., Olsen, R., Bryant, A., Homanics, G., Firestone, L., Skinner, S., Handforth, A., Asataourian, A. & Delgado-Escueta, A. (1996) Am. J. Hum. Genet. 59, Suppl., A273 (abstr.).
- 12.Nagy A, Cocza E, Merenties Diaz E, Prideaux V R, Ivanyi E, Markkula M, Rossant J. Development (Cambridge, UK) 1990;110:815–821. doi: 10.1242/dev.110.3.815. [DOI] [PubMed] [Google Scholar]
- 13.Huh K H, Delorey T M, Endo S, Olsen R W. Mol Pharmacol. 1995;48:666–675. [PubMed] [Google Scholar]
- 14.Quinlan J J, Firestone L L. Pharmacol Biochem Behav. 1992;42:787–790. doi: 10.1016/0091-3057(92)90030-j. [DOI] [PubMed] [Google Scholar]
- 15.Alifimoff J, Firestone L, Miller K. Anesthesiology. 1987;66:55–59. doi: 10.1097/00000542-198701000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Olsen R W, McCabe R T, Wamsley J K. J Chem Neuroanat. 1990;3:59–76. [PubMed] [Google Scholar]
- 17.Korpi E R, Kleingoor C, Kettenmann H, Seeburg P H. Nature (London) 1993;361:356–359. doi: 10.1038/361356a0. [DOI] [PubMed] [Google Scholar]
- 18.Korpi E, Seeburg P, Luddens H. J Neurochem. 1996;66:2179–2187. doi: 10.1046/j.1471-4159.1996.66052179.x. [DOI] [PubMed] [Google Scholar]
- 19.White G. Brain Res. 1992;585:56–62. doi: 10.1016/0006-8993(92)91190-p. [DOI] [PubMed] [Google Scholar]
- 20.Koltchine V, Ye Q, Finn S, Harrison N. Neuropharmacology. 1996;35:1445–1456. doi: 10.1016/s0028-3908(96)00088-3. [DOI] [PubMed] [Google Scholar]
- 21.Lyon M F, King T R, Gondo Y, Gardner J M, Nakatsu Y, Eicher E M, Brilliant M H. Proc Natl Acad Sci USA. 1992;89:6968–6972. doi: 10.1073/pnas.89.15.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Culiat C T, Stubbs L, Nicholls R D, Montgomery C S, Russell L B, Johnson D K, Rinchik R M. Proc Natl Acad Sci USA. 1993;90:5105–5109. doi: 10.1073/pnas.90.11.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abbott B D, Birnbaum L S. Teratology. 1990;42:597–610. doi: 10.1002/tera.1420420604. [DOI] [PubMed] [Google Scholar]
- 24.Abbott B D, Birnbaum L S. Toxicol Appl Pharmacol. 1990;106:418–432. doi: 10.1016/0041-008x(90)90337-t. [DOI] [PubMed] [Google Scholar]
- 25.Fraser F C. Am J Hum Genet. 1989;45:345–347. [PMC free article] [PubMed] [Google Scholar]
- 26.Murray J C. Am J Hum Genet. 1995;57:227–232. [PMC free article] [PubMed] [Google Scholar]
- 27.Zimmerman E F. Prog Clin Biol Res. 1985;171:283–294. [PubMed] [Google Scholar]
- 28.Miller R P, Becker B A. Toxicol Appl Pharmacol. 1975;32:53–61. doi: 10.1016/0041-008x(75)90194-5. [DOI] [PubMed] [Google Scholar]
- 29.Pritchett D B, Seeburg P H. J Neurochem. 1990;54:1802–1805. doi: 10.1111/j.1471-4159.1990.tb01237.x. [DOI] [PubMed] [Google Scholar]
- 30.Lüddens H, Seeburg P H, Korpi E R. Mol Pharmacol. 1994;45:810–814. [PubMed] [Google Scholar]
- 31.Benke D, Fritschy J M, Trzeciak A, Bannwarth W, Mohler H. J Biol Chem. 1994;269:27100–27107. [PubMed] [Google Scholar]
- 32.Ma W, Saunders P A, Somogyi R, Poulter M O, Barker J L. J Comp Neurol. 1993;338:337–359. doi: 10.1002/cne.903380303. [DOI] [PubMed] [Google Scholar]
- 33.Wingrove P B, Wafford K A, Bain C, Whiting P J. Proc Natl Acad Sci USA. 1994;91:4569–4573. doi: 10.1073/pnas.91.10.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eccles J, Eccles R, Magni F. J Physiol (London) 1961;159:147–166. doi: 10.1113/jphysiol.1961.sp006798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rise M L, Frankel W N, Coffin J M, Seyfried T N. Science. 1991;253:669–673. doi: 10.1126/science.1871601. [DOI] [PubMed] [Google Scholar]
- 36.Guerrini R, DeLorey T, Bonanni P, Moncla A, Dravet C, Suisse G, Livet M, Bureau M, Malzac P, Genton P, Thomas P, Sartucci F, Simi P, Serratosa J. Ann Neurol. 1996;39:699–708. doi: 10.1002/ana.410400109. [DOI] [PubMed] [Google Scholar]
- 37.Angelman H. Dev Med Child Neurol. 1965;7:681–668. [Google Scholar]
- 38.Williams C A, Angelman H, Clayton-Smith J, Driscoll D J, Hendrickson J E, Knoll J H, Magenis R E, Schinzel A, Wagstaff J, Whidden E M, Zori R T. Am J Med Genet. 1995;56:237–238. doi: 10.1002/ajmg.1320560224. [DOI] [PubMed] [Google Scholar]
- 39.Saitoh S, Kubota T, Ohta T, Jinno Y, Niikawa N, Sugimoto T, Wagstaff J, Lalande M. Lancet. 1992;339:366–367. doi: 10.1016/0140-6736(92)91686-3. [DOI] [PubMed] [Google Scholar]