Abstract
Chemical coding in the sympathetic nervous system involves both noradrenergic and, for a minority of neurons, cholinergic neurotransmission. The expression of the cholinergic phenotype in the developing sympathetic nervous system was examined to determine if coding for cholinergic transmission occurs before or after innervation of peripheral target organs. The vesicular acetylcholine transporter (VAChT) and choline acetyltransferase, the products of the “cholinergic gene locus” determining the cholinergic phenotype, were expressed in principal cells of the paravertebral, but only rarely in prevertebral, sympathetic chains as early as embryonic day 14. A subpopulation of VAChT- and choline acetyltransferase-positive sympathetic ganglion cells persisted throughout development of the stellate and more caudal paravertebral ganglia into anatomically distinct cell groups, and into adulthood. The forepaw eccrine sweat glands, innervated exclusively by the stellate ganglion, received VAChT-positive nerve terminals at least as early as postembryonic day 4, coincident with the development of the sweat glands themselves. These terminals, like the VAChT-positive cell bodies of the developing stellate ganglion, have some noradrenergic traits including expression of tyrosine hydroxylase, but did not express the vesicular monoamine transporter, and are therefore not functionally noradrenergic. Development of the cholinergic phenotype in principal cells of the sympathetic paravertebral ganglia apparently occurs via receipt of instructive cues, or selection, within the sympathetic chain itself or perhaps even during migration of the cells of the neural crest from which the paravertebral ganglia arise.
Keywords: autonomic nervous system development, tyrosine hydroxylase, vesicular acetylcholine transporter, vesicular monoamine transporter
The majority of the postganglionic neurons of the sympathetic nervous system, including those innervating peripheral targets such as the cardiovascular system, fat-storing tissues, and visceral organs are noradrenergic. Some, innervating the eccrine sweat glands and skeletal muscle vasculature, are cholinergic. How the cholinergic and noradrenergic sympathetic phenotypes arise to allow precise chemical coding of neurotransmission at their respective synaptic targets is an important neurodevelopmental question. Principal ganglion cells, at least in the chicken, express the catecholamine biosynthetic enzyme tyrosine hydroxylase (TH) during embryonic development after commitment of their neural crest progenitors to a neuronal phenotype, but before innervation of their peripheral targets (1, 2). Mesencephalic neural crest cells assumed to give rise to postganglionic parasympathetic neurons express the cholinergic marker choline acetyltransferase already during neural crest migration (3).
Based on studies in the developing sweat gland, which is a target of sympathetic cholinergic innervation in the adult rat, the cholinergic sympathetic phenotype has been hypothesized to arise postnatally in neurons that initially express the noradrenergic phenotype (4, 5). However, direct examination of the onset of expression of both components of the “cholinergic gene locus,” vesicular acetylcholine transporter (VAChT) and choline acetyltransferase (ChAT), required for cholinergic neurotransmission, in the developing sympathetic nervous system, has not been reported.
Here, specific antibodies against VAChT and vesicular monoamine transporter 2 (VMAT2), and in situ hybridization histochemistry for expression of the products of the cholinergic gene locus, VAChT and ChAT, have been used to demonstrate that the cholinergic phenotype exists during early embryonic development of the sympathetic chain from the neural crest, and that neurons that arise from the sympathetic chain and innervate the sweat gland are already cholinergic at the time of innervation. In addition, these neurons have been examined for expression of the noradrenergic vesicular transporter, VMAT2, to determine if during development they exhibit a full noradrenergic as well as a cholinergic chemical phenotype.
MATERIALS AND METHODS
Immunohistochemistry.
For immunohistochemistry, rat embryos of the appropriate developmental age as well as individual tissues were immersion fixed in Bouins’ solution containing 6% (wt/vol) picric acid, 2.5% cupric acetate, 3.7% formaldehyde, and 1% glacial acetic acid. Specimens were fixed for 1–2 days (depending on tissue mass) at room temperature, dehydrated, and mounted in paraffin. Sections were cut at 7–10 μm, placed on silanized glass slides, and prepared for immunohistochemical analyses as described elsewhere (6). Briefly, sections were rehydrated, and endogenous peroxidase activity blocked by incubation in 3% H2O2 in methanol for 30 min. Sections were washed twice in distilled water for 5 min, immersed in 0.01 M sodium citrate (pH 6) preheated to 95°C and incubated at 95°C for 10 min to enhance immunohistochemical staining. Nonspecific antibody binding was blocked by incubation in 5% BSA in PBS for 30 min. After another wash step sections were incubated with primary antibody (anti-VAChT #80259 at 1:1500; anti-VMAT2 #80182 at 1:1500; refs. 7–9) overnight at 16°C and 2 hr at 37°C. Sections were washed again and incubated with a biotinylated secondary antibody for 45 min at 37°C. Final incubation with an avidin-biotinylated horseradish peroxidase complex for 2 hr at 37°C was followed by multiple washes as above, and visualization of the immobilized peroxidase by reaction with diaminobenzidine/nickel in the presence of 0.003% H2O2 to form an insoluble black reaction product. Sections were washed in water, dehydrated with graded ethanol/xylene, and mounted with Depex under glass coverslips.
In Situ Hybridization Histochemistry.
Tissues were snap frozen in isopentane at −50°C, and stored at −80°C until cryosectioning at 14–16 μm. Sections were placed on Star-Frost presilanized glass slides, dried at room temperature, and prehybridized (10). Sections were hybridized with 35S-labeled ribonucleotide probes, washed, coated with emulsion, and developed and viewed as described (10). A rat ChAT riboprobe was generated by PCR amplification of a ChAT cDNA target obtained by reverse transcription of rat spinal cord mRNA and amplification of self-primed double-stranded DNA using sense and antisense primers from nucleotides 134–160 and 380–404 of rat ChAT, respectively, to yield a 270-bp DNA fragment that was subsequently subcloned into pGEM-T (Promega), and sequence confirmed by double-stranded sequencing.
RESULTS AND DISCUSSION
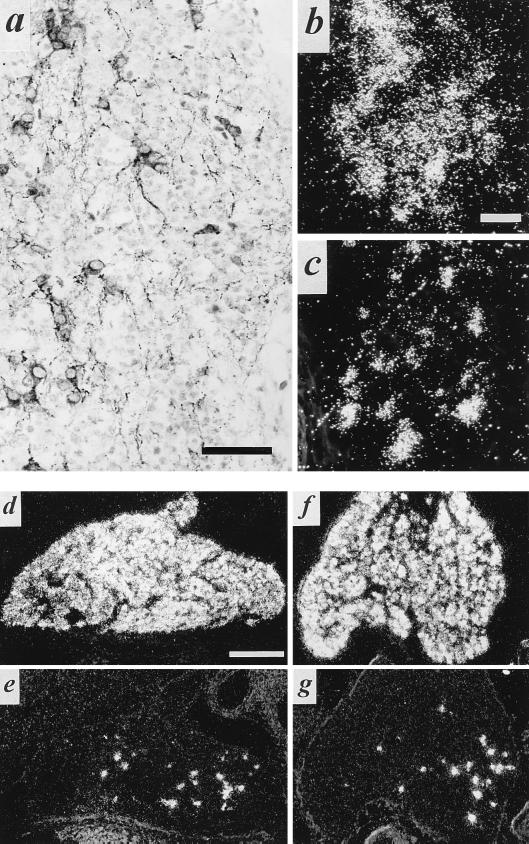
The vesicular acetylcholine and monoamine transporters are highly specific and sensitive immunohistochemical markers for cholinergic and noradrenergic cell bodies and nerve terminals in the autonomic nervous system (7, 8). The availability of specific polyclonal antibodies for VAChT, in particular, affords sensitive and direct visualization of the development of both cholinergic postganglionic sympathetic neurons and their terminal fields in vivo. VAChT mRNA and protein were expressed at embryonic day 14 (E14) in a subpopulation of principal cells within the paravertebral sympathetic chain (Fig. 1). Only rarely did principal cells of the prevertebral chain express VAChT during development as expected from the virtual absence of cholinergic sympathetic neurons in this component of the autonomic nervous system in the adult animal.
Figure 1.
Expression of VMAT2 and VAChT in developing pre- and paravertebral ganglia of the rat. Expression of VMAT2 (a) and VAChT (b) mRNA in E14 sympathetic paravertebral (Left) and prevertebral (Right) chains detected by in situ hybridization histochemistry. Expression of VMAT2 (c) and VAChT (d) protein in E14 sympathetic paravertebral (Left) and prevertebral (Right) chains detected by immunohistochemistry. Note that the majority of principal ganglion cells in both pre- and paravertebral ganglia express VMAT2 at this stage of development. In contrast, VAChT expression is observed in several cells of the paravertebral, but only rarely in the prevertebral sympathetic chain. Note (d) the copious VAChT immunoreactivity investing principal ganglion cells of both pre- and paravertebral ganglia, and representing preganglionic cholinergic innervation of these cells, at E14. [Bar in b = 250 μm and this magnification holds for all micrographs in this figure.]
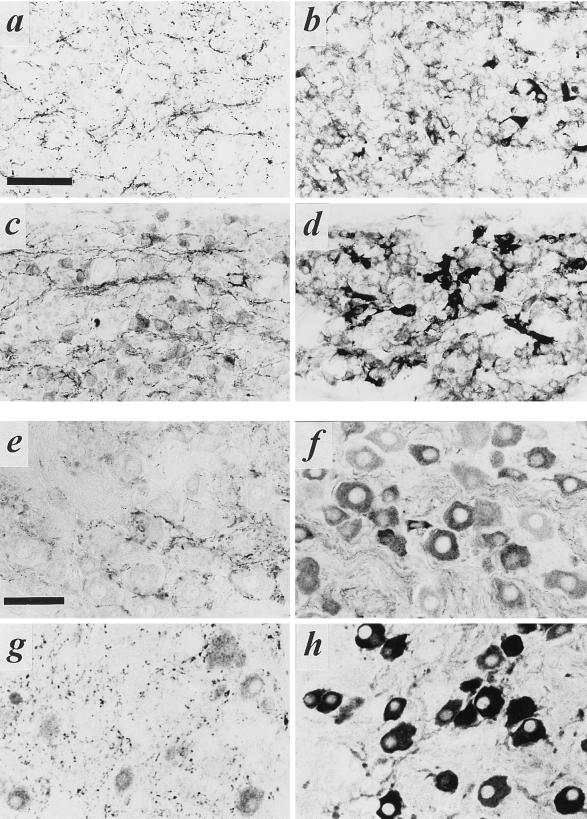
The VAChT-positive subpopulation of principal cells in the paravertebral chain persisted through E16, at which time the sympathetic chain has become organized into distinct groups of principal cells, or ganglia. The number of VAChT-positive cells in the stellate ganglion was much higher than that of the superior cervical ganglion, both during development at day E16 (Fig. 2 a–d) and in the adult rat (Fig. 2 e–h). This is consistent with similar data obtained with anti-ChAT antibodies in adult rat stellate and superior cervical ganglia (11).
Figure 2.
VAChT and VMAT2 expression in embryonic and adult superior cervical and stellate sympathetic ganglia. (a–d) Staining of superior cervical (a and b) and stellate (c and d) ganglia for VAChT (a and c) or VMAT2 (b and d) on E16. Note copious cholinergic preganglionic terminal immunoreactivity in both ganglia at this stage of development, large numbers of VMAT2-positive principal ganglion cells in both ganglia, and frequent VAChT-positive principal ganglion cells in stellate (c) but not superior cervical (a) ganglion at this time. (e–h) Staining of superior cervical (e and f) and stellate (g and h) ganglia for VAChT (e and g) or VMAT2 (f and h) in adult. Note copious cholinergic preganglionic terminal immunoreactivity in both ganglia, large numbers of VMAT2-positive principal ganglion cells in both ganglia, and frequent VAChT-positive principal ganglion cells in stellate (g) but not superior cervical (e) ganglion at this time. (Bars in a and e = 50 μm; all panels at same magnification.)
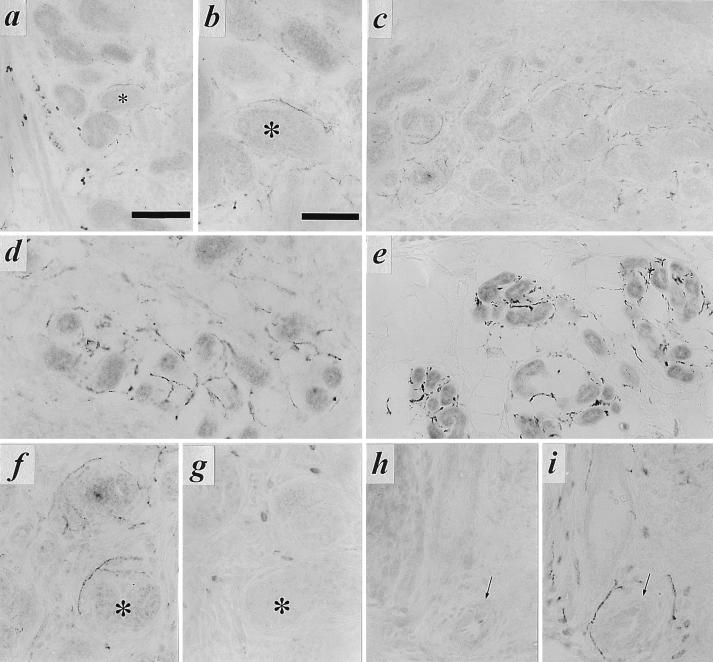
VAChT and ChAT are the products of the cholinergic gene locus that impart a functional cholinergic phenotype to neuronal cells (12, 13). The number of stellate ganglion neurons that are cholinergic by this criterion appeared to be relatively constant from the latter half of gestation into adulthood based on analysis of random sections of the ganglion by in situ hybridization and immunohistochemistry for VAChT throughout development (Fig. 3). Thus, the expression of the cholinergic phenotype in the stellate ganglion during development is not a transient phenomenon. Rather, the stellate ganglion, as well as thoracic and lumbar paravertebral ganglia (data not shown), appeared to be endowed with their full complement of cholinergic neurons prior to birth, and thus well before innervation of sympathetic cholinergic targets such as the sweat gland.
Figure 3.
VAChT/ChAT expression in a subpopulation of principal cells of the developing stellate ganglion. (a) Immunohistochemistry for VAChT in stellate ganglion at E19. Note numerous VAChT-positive principal cells as in Fig. 2, as well as VAChT-positive preganglionic terminals at this stage of development. (b and c) Detection of VAChT mRNA (b) and ChAT mRNA (c) in E19 stellate ganglion. Exposure for 14 days following in situ hybridization. Note b and c represent nonadjacent sections from the same ganglion. (d and e) VMAT2 (d) and VAChT (e) in situ hybridization histochemistry, postnatal day 2 (P2). (f and g) VMAT2 (f) and VAChT (g) in situ hybridization histochemistry, P11. Note relative constancy in the number of VAChT mRNA-positive cells per section of whole ganglion throughout development at E19 (b), P2 (e), and P11 (g). [Bars = 50 μm (a), 10 μm (b and c), and 250 μm (d–g).]
Prenatal expression of VAChT and ChAT in the developing sympathetic chain, and in particular in the stellate ganglion, was a surprising observation. It suggested that cholinergic neurons per se might directly innervate their target tissues in vivo, without the requirement for functional noradrenergic innervation followed by a target-dependent switch to the cholinergic phenotype (14). Accordingly, we examined the pattern of expression within the developing sweat gland of the VMAT2 neuronal isoform of the vesicular monoamine transporter and VAChT. Use of these neurotransmitter-specific nerve terminal markers allowed sensitive and high-resolution light microscopic examination of noradrenergic and cholinergic terminals in the sweat gland (8).
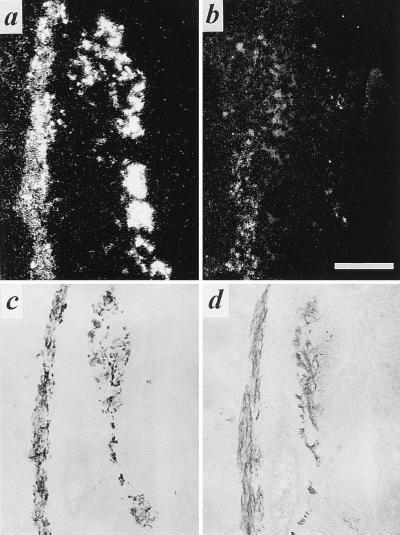
The presence of VMAT2 in noradrenergic neurons innervating blood vessels and of VAChT in developing sweat gland coils of the rat forepaw are shown in Fig. 4. Appearance of sympathetic cholinergic innervation of the sweat glands was coincident with development of sweat gland secretory coils within the footpad during early postnatal life. It developed from an initial sparse but fully VAChT-positive innervation at postnatal day four to a mature pattern of cholinergic innervation of the sweat glands by P11 (Fig. 4 a–e). In addition, these neurons, in contrast to those innervating nearby blood vessels at all postnatal times, were VMAT2-negative during the period in which innervation reaches mature levels, shown here at P8 (Fig. 4 f–i).
Figure 4.
Expression of VAChT in nerve terminals innervating developing sweat glands in early postnatal development, and mutual exclusivity of VMAT2 and VAChT immunoreactivity in footpad nerve terminals. (a) Immunohistochemical staining for VAChT (as described in legend to Fig. 1) in rat sweat gland tissue at P4. Note intense VAChT-positive neuromuscular junctions within skeletal muscle at bottom left. Faint but distinct terminal staining can be seen on most of the sweat gland epithelial cell clusters, beginning to coil into sweat glands proper at P4. Nonspecific staining of blood cells is seen within lumen of large vessel separating skeletal muscle and sweat gland epithelial regions. Asterisk marks sweat gland seen at high power in b. (b) VAChT-positive terminals at P4, high magnification. Note VAChT-immunoreactivity in terminals closely contacting developing secretory coils of developing eccrine sweat glands. (c–e) VAChT-positive terminals in P8 (c), P11 (d), and adult (e) forepaw sweat glands. Note increasing density of cholinergic terminals through the first two postnatal weeks. (f–i) VAChT (f and h) and VMAT2 (g and i) in adjacent sections (f and g; h and i) of P8 sweat gland. Asterisk in f and g marks sweat gland positive for VAChT (f) and negative for VMAT2 in adjacent section (g). Arrows in h and i mark blood vessel negative for VAChT (h) and positive for VMAT2 (i). VAChT immunoreactivity in sweat gland nerve terminals was shown to be specific by complete loss of immunoreactivity upon preincubation of anti-VAChT antibody, at its final working dilution, with the C-terminal VAChT peptide against which the antibody was raised, at a final concentration of 10 μM. VMAT2 immunostaining was unaffected by preincubation with this concentration of the VAChT peptide. [Bars = 100 μm (a, c, and e) and 50 μm (b, d, and f–i).]
The other isoform of the vesicular monoamine transporter, VMAT1, is absent from neurons and selectively expressed in neuroendocrine cells, in the adult rat (7, 15). However, VMAT1 is transiently coexpressed with VMAT2 in sympathetic ganglia of the rat during development (16). Thus it, rather than VMAT2, could be the noradrenergic vesicular transporter expressed in cholinergic sympathetic neurons innervating the sweat gland, potentially endowing these neurons with a functional noradrenergic as well as a cholinergic chemical phenotype. VMAT1 immunoreactivity was absent from nerve fibers innervating the forepaw sweat glands at all postnatal days examined, eliminating this possibility (data not shown).
TH immunoreactivity has been observed to be present in neurons innervating the sweat gland epithelium proper, from postnatal days 2–10, and is progressively lost by three weeks postnatally (17). We confirmed this observation in forepaw sweat glands during the first postnatal week (data not shown), which suggests that cholinergic (VAChT+) nerve terminals of the sweat gland initially possess only a partial (TH+/VMAT2−) noradrenergic phenotype. A high-affinity norepinephrine (NE) uptake carrier is probably also expressed on cholinergic sweat gland neurons during early embryonic life, accounting for loss of cholinergic sweat gland innervation when 6-hydroxydopamine is administered early but not later in development (17). Thus, expression of functional components of the noradrenergic phenotype specific to noradrenergic neurons, such as TH and possibly the high-affinity NE plasma membrane carrier (NET), are under a different type of developmental control in sympathetic neurons in vivo than functional components of the noradrenergic phenotype common to all monoaminergic neurons, such as VMAT2.
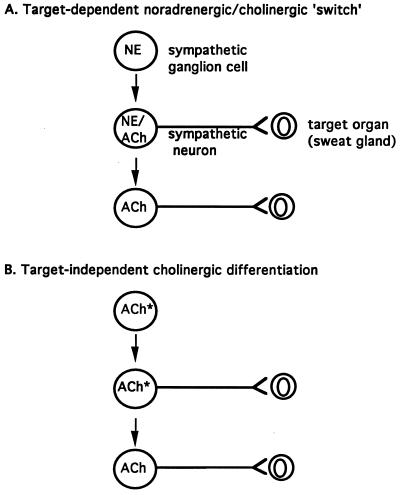
Does innervation per se attenuate expression of noradrenergic components of a noradrenergic/cholinergic dual phenotype at the level of the neuronal cell body, via transmission of a retrograde signal from the sweat gland to the stellate ganglion? The establishment of the cholinergic phenotype, or at least transcription from the VAChT/ChAT cholinergic gene locus, appears not to be target-dependent. However, loss of components of the noradrenergic phenotype such as TH and the NE plasma membrane transporter from sweat gland cholinergic sympathetic neurons during development may well be. Existing data and those presented here favor a model like that depicted in Fig. 5B, rather than the model depicted in Fig. 5A, for cholinergic differentiation in the sympathetic nervous system. Target independence of differentiation of cholinergic principal ganglion cells in the sympathetic nervous system suggests that apportioning of cholinergic and noradrenergic phenotypes in the paravertebral sympathetic chain may occur upon reception of positional cues received from nearby tissues during embryonic development. Consistent with this possibility, a substantially lower percentage of cholinergic principal cells exists in the superior cervical compared with the stellate ganglion of the rat at all stages of pre- and postnatal development (see Fig. 2). Such a mechanism suggests cholinergic sympathetic development occurs as a part of autonomic neuronal differentiation within the sympathetic chain (2, 18).
Figure 5.
Models for postganglionic cholinergic neuronal differentiation in the sympathetic nervous system. (A) Sympathetic cholinergic neurons arise from principal ganglion cells via formation of fully functional noradrenergic neurons (NE) lacking cholinergic expression that synapse upon targets such as the sweat glands and induce the secretion from the target of factor(s) that induce the cholinergic phenotype and attenuate the expression of noradrenergic traits in the noradrenergic neuron (see ref. 5). (B) A proportion of the principal ganglion cells in the sympathetic chain express the cholinergic phenotype prior to innervation of target organs. Asterisk indicates a cholinergic (i.e., ChAT+/VAChT+) phenotype with noradrenergic [e.g., TH, dopamine β-hydroxylase (DBH), NET, but not VMAT] traits. Loss of noradrenergic traits in rat postganglionic cholinergic sympathetic neurons may occur as a direct result of interaction with the target, or may also be programmed in a target-independent manner.
A neurotransmitter-dependent switch from a fully noradrenergic to a functionally cholinergic phenotype occurs in neonatal superior cervical ganglion principal cells cultured in the presence of sweat gland epithelia (19, 20). The absence of such a mechanism in vivo may reflect intrinsic differences in the phenotypic potential of cells of the stellate and superior cervical ganglia at this late stage in development, with the cholinergic potential of the stellate ganglion being realized early and more intensely in vivo, and that of the superior cervical ganglion remaining latent but susceptible to phenotypic alteration in vitro. The role of targets such as the sweat gland in postnatal attenuation of the noradrenergic traits expressed in cholinergic sympathetic neurons continues to be an open question, as is the actual time of development of patent cholinergic transmission in these neurons and its role in target tissue development (21). Cholinergic differentiation factors, i.e., those allowing transcription from the VAChT/ChAT gene locus, apparently must act within the milieu of the developing sympathetic ganglia themselves. Peripheral tissue targets, on the other hand, might supply factors that restrict expression of noradrenergic traits and permit the further development of functional cholinergic neurotransmission.
Acknowledgments
We thank Petra Lattermann, Elke Rodenberg, Heike Reichert-Preibsch, Silke Roscher, and Joerg Schmidt for excellent technical assistance and Heidemarie Schneider for photographic documentation. This work was supported in part by the German Research, Volkswagen, and Kempkes Foundations, and the National Institute of Mental Health Intramural Research Program.
ABBREVIATIONS
- ACh
acetylcholine
- ChAT
choline acetyltransferase
- NE
norepinephrine
- En
embryonic day
- Pn
postembryonic day
- TH
tyrosine hydroxylase
- VAChT
vesicular acetylcholine transporter
- VMAT
vesicular monoamine transporter
- NET
NE transporter
References
- 1.Groves A K, George K M, Tissier-Seta J-P, Engel J D, Brunet J-F, Anderson D J. Development (Cambridge, UK) 1995;12:887–901. doi: 10.1242/dev.121.3.887. [DOI] [PubMed] [Google Scholar]
- 2.Reissmann E, Ernsberger U, Francis-West P H, Rueger D, Brickell P M, Rohrer H. Development (Cambridge, UK) 1996;122:2079–2088. doi: 10.1242/dev.122.7.2079. [DOI] [PubMed] [Google Scholar]
- 3.Smith J, Fauquet M, Ziller C, Le Douarin N M. Nature (London) 1979;282:853–855. doi: 10.1038/282853a0. [DOI] [PubMed] [Google Scholar]
- 4.Landis S C, Keefe D. Dev Biol. 1983;98:349–372. doi: 10.1016/0012-1606(83)90365-2. [DOI] [PubMed] [Google Scholar]
- 5.Landis S C. Trends Neurosci. 1990;13:344–350. doi: 10.1016/0166-2236(90)90147-3. [DOI] [PubMed] [Google Scholar]
- 6.Schäfer M K-H, Nohr D, Romeo H, Eiden L E, Weihe E. Peptides (Tarrytown, NY) 1994;15:263–279. doi: 10.1016/0196-9781(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 7.Weihe E, Schäfer M K-H, Erickson J D, Eiden L E. J Mol Neurosci. 1994;5:149–164. doi: 10.1007/BF02736730. [DOI] [PubMed] [Google Scholar]
- 8.Weihe E, Tao-Cheng J-H, Schäfer M K-H, Erickson J D, Eiden L E. Proc Natl Acad Sci USA. 1996;93:3547–3552. doi: 10.1073/pnas.93.8.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erickson J D, Schäfer M K-H, Bonner T I, Eiden L E, Weihe E. Proc Natl Acad Sci USA. 1996;93:5166–5171. doi: 10.1073/pnas.93.10.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schäfer M K-H, Weihe E, Varoqui H, Eiden L E, Erickson J D. J Mol Neurosci. 1994;5:1–18. doi: 10.1007/BF02736691. [DOI] [PubMed] [Google Scholar]
- 11.Morales M A, Holmberg K, Xu Z Q, Cozzari C, Hartman B K, Emson P, Goldstein M, Elfvin L-G, Hökfelt T. Proc Natl Acad Sci USA. 1995;92:11819–11823. doi: 10.1073/pnas.92.25.11819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erickson J D, Varoqui H, Schäfer M, Diebler M-F, Weihe E, Modi W, Rand J, Eiden L E, Bonner T I, Usdin T. J Biol Chem. 1994;269:21929–21932. [PubMed] [Google Scholar]
- 13.Usdin T, Eiden L E, Bonner T I, Erickson J D. Trends Neurosci. 1995;18:218–224. doi: 10.1016/0166-2236(95)93906-e. [DOI] [PubMed] [Google Scholar]
- 14.Landis S C. Prog Brain Res. 1994;100:19–23. doi: 10.1016/s0079-6123(08)60763-3. [DOI] [PubMed] [Google Scholar]
- 15.Peter D, Liu Y, Sternini C, de Giorgio R, Brecha N, Edwards R H. J Neurosci. 1995;15:6179–6188. doi: 10.1523/JNEUROSCI.15-09-06179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schütz, B., Schäfer, M. K.-H., Eiden, L. E. & Weihe, E. (1997) Adv. Pharmacol., in press. [DOI] [PubMed]
- 17.Landis S C. In: Neurotransmitter Plasticity in Sympathetic Neurons and Its Regulation By Environmental Factors in Vitro and in Vivo. Björklund A, Hökfelt T, Owman C, editors. Vol. 6. Amsterdam: Elsevier; 1988. pp. 65–115. [Google Scholar]
- 18.Shah N M, Groves A K, Anderson D J. Cell. 1996;85:331–343. doi: 10.1016/s0092-8674(00)81112-5. [DOI] [PubMed] [Google Scholar]
- 19.Landis S C. Dev Biol. 1980;77:348–361. doi: 10.1016/0012-1606(80)90480-7. [DOI] [PubMed] [Google Scholar]
- 20.Habecker B A, Landis S C. Science. 1994;264:1602–1604. doi: 10.1126/science.8202714. [DOI] [PubMed] [Google Scholar]
- 21.Grant M P, Francis N J, Landis S C. Mol Cell Neurosci. 1995;6:32–42. doi: 10.1006/mcne.1995.1004. [DOI] [PubMed] [Google Scholar]