Abstract
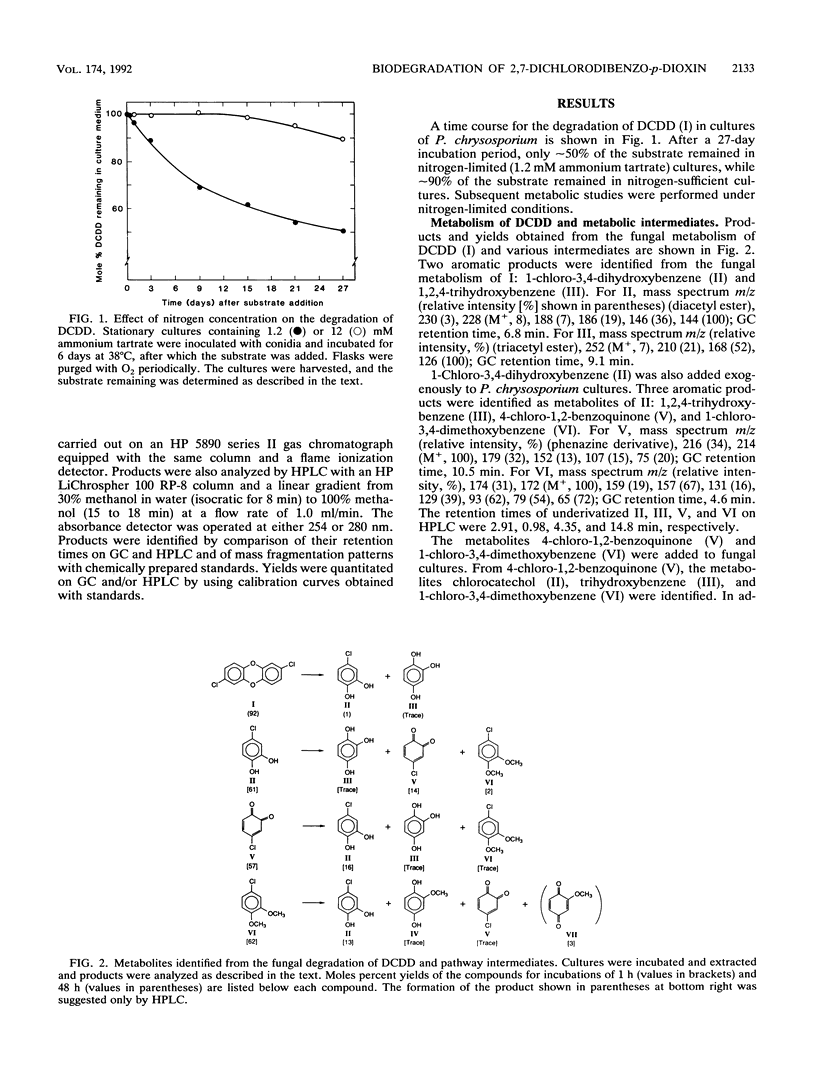
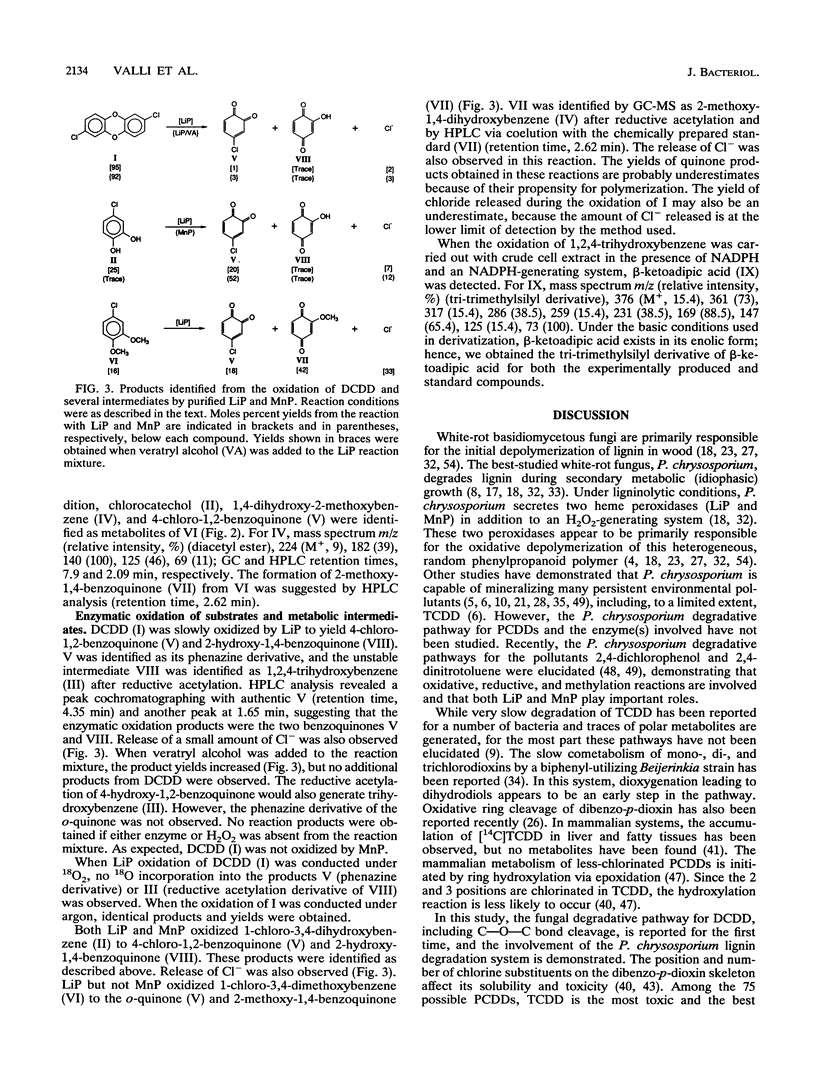
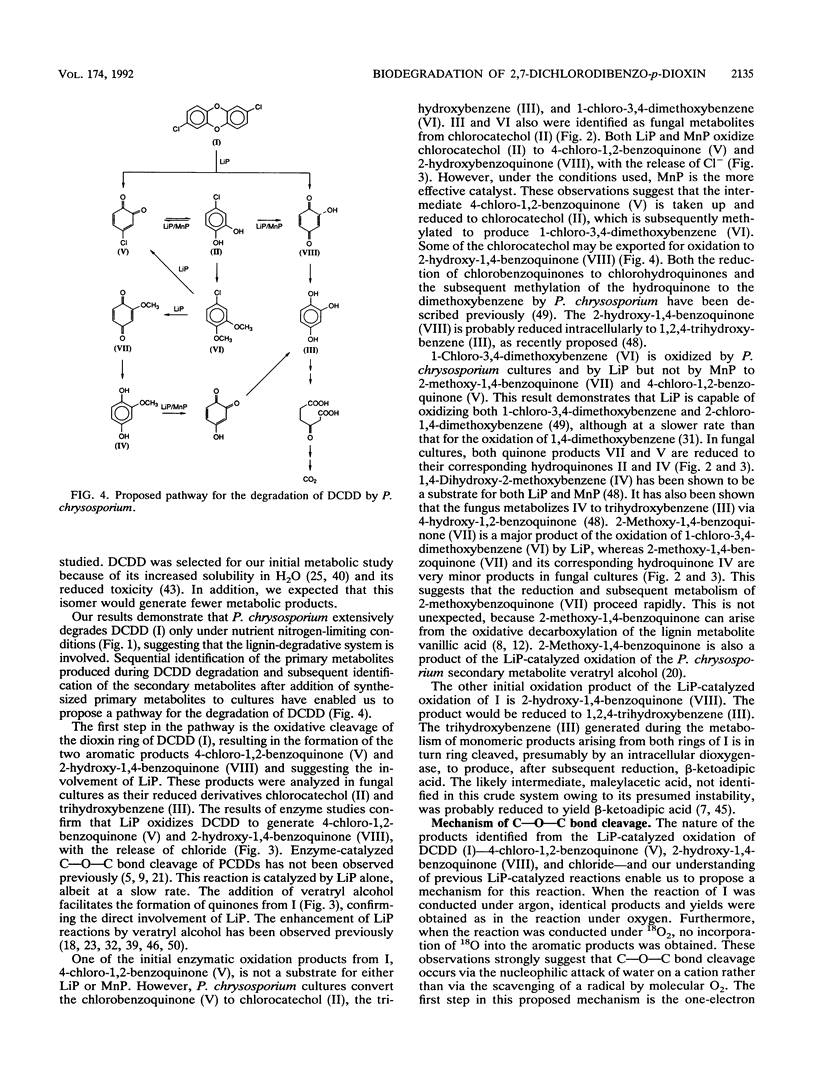
Under secondary metabolic conditions, the white-rot basidiomycete Phanerochaete chrysosporium degraded 2,7-dichlorodibenzo-p-dioxin (I). The pathway for the degradation of I was elucidated by the characterization of fungal metabolites and oxidation products generated by lignin peroxidase (LiP), manganese peroxidase (MnP), and crude intracellular cell-free extracts. The multistep pathway involves the degradation of I and subsequent intermediates by oxidation, reduction, and methylation reactions to yield the key intermediate 1,2,4-trihydroxybenzene (III). In the first step, the oxidative cleavage of the dioxin ring of I, catalyzed by LiP, generates 4-chloro-1,2-benzoquinone (V), 2-hydroxy-1,4-benzoquinone (VIII), and chloride. The intermediate V is then reduced to 1-chloro-3,4-dihydroxybenzene (II), and the latter is methylated to form 1-chloro-3,4-dimethoxybenzene (VI). VI in turn is oxidized by LiP to generate chloride and 2-methoxy-1,4-benzoquinone (VII), which is reduced to 2-methoxy-1,4-dihydroxybenzene (IV). IV is oxidized by either LiP or MnP to generate 4-hydroxy-1,2-benzoquinone, which is reduced to 1,2,4-trihydroxybenzene (III). The other aromatic product generated by the initial LiP-catalyzed cleavage of I is 2-hydroxy-1,4-benzoquinone (VIII). This intermediate is also generated during the LiP- or MnP-catalyzed oxidation of the intermediate chlorocatechol (II). VIII is also reduced to 1,2,4-trihydroxybenzene (III). The key intermediate III is ring cleaved by intracellular cell extracts to produce, after reduction, beta-ketoadipic acid. In this pathway, initial oxidative cleavage of both C-O-C bonds in I by LiP generates two quinone products, 4-chloro-1,2-benzoquinone (V) and 2-hydroxy-1,4-benzoquinone (VIII). The former is recycled by reduction and methylation reactions to generate an intermediate which is also a substrate for peroxidase-catalyzed oxidation, leading to the removal of a second chlorine atom. This unique pathway results in the removal of both aromatic chlorines before aromatic ring cleavage takes place.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alic M., Letzring C., Gold M. H. Mating System and Basidiospore Formation in the Lignin-Degrading Basidiomycete Phanerochaete chrysosporium. Appl Environ Microbiol. 1987 Jul;53(7):1464–1469. doi: 10.1128/aem.53.7.1464-1469.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman R., Meselson M. An analytical method for detecting TCDD (dioxin): levels of TCDD in samples from Vietnam. Environ Health Perspect. 1973 Sep;5:27–35. doi: 10.1289/ehp.730527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boominathan K., Dass S. B., Randall T. A., Kelley R. L., Reddy C. A. Lignin peroxidase-negative mutant of the white-rot basidiomycete Phanerochaete chrysosporium. J Bacteriol. 1990 Jan;172(1):260–265. doi: 10.1128/jb.172.1.260-265.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumpus J. A., Tien M., Wright D., Aust S. D. Oxidation of persistent environmental pollutants by a white rot fungus. Science. 1985 Jun 21;228(4706):1434–1436. doi: 10.1126/science.3925550. [DOI] [PubMed] [Google Scholar]
- Commandeur L. C., Parsons J. R. Degradation of halogenated aromatic compounds. Biodegradation. 1990;1(2-3):207–220. doi: 10.1007/BF00058837. [DOI] [PubMed] [Google Scholar]
- Glenn J. K., Gold M. H. Purification and characterization of an extracellular Mn(II)-dependent peroxidase from the lignin-degrading basidiomycete, Phanerochaete chrysosporium. Arch Biochem Biophys. 1985 Nov 1;242(2):329–341. doi: 10.1016/0003-9861(85)90217-6. [DOI] [PubMed] [Google Scholar]
- Gold M. H., Kuwahara M., Chiu A. A., Glenn J. K. Purification and characterization of an extracellular H2O2-requiring diarylpropane oxygenase from the white rot basidiomycete, Phanerochaete chrysosporium. Arch Biochem Biophys. 1984 Nov 1;234(2):353–362. doi: 10.1016/0003-9861(84)90280-7. [DOI] [PubMed] [Google Scholar]
- Guenthner T. M., Fysh J. M., Nebert D. W. 2,3,7,8-Tetrachlorodibenzo-p-dioxin: covalent binding of reactive metabolic intermediates principally to protein in vitro. Pharmacology. 1979;19(1):12–22. doi: 10.1159/000137278. [DOI] [PubMed] [Google Scholar]
- Hammel K. E., Kalyanaraman B., Kirk T. K. Oxidation of polycyclic aromatic hydrocarbons and dibenzo[p]-dioxins by Phanerochaete chrysosporium ligninase. J Biol Chem. 1986 Dec 25;261(36):16948–16952. [PubMed] [Google Scholar]
- Harms H., Wittich R. M., Sinnwell V., Meyer H., Fortnagel P., Francke W. Transformation of Dibenzo-p-Dioxin by Pseudomonas sp. Strain HH69. Appl Environ Microbiol. 1990 Apr;56(4):1157–1159. doi: 10.1128/aem.56.4.1157-1159.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten P. J., Tien M., Kalyanaraman B., Kirk T. K. The ligninase of Phanerochaete chrysosporium generates cation radicals from methoxybenzenes. J Biol Chem. 1985 Mar 10;260(5):2609–2612. [PubMed] [Google Scholar]
- Kirk T. K., Farrell R. L. Enzymatic "combustion": the microbial degradation of lignin. Annu Rev Microbiol. 1987;41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- Klecka G. M., Gibson D. T. Metabolism of Dibenzo-p-Dioxin and Chlorinated Dibenzo-p- Dioxins by a Beijerinckia Species. Appl Environ Microbiol. 1980 Feb;39(2):288–296. doi: 10.1128/aem.39.2.288-296.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamar R. T., Larsen M. J., Kirk T. K. Sensitivity to and Degradation of Pentachlorophenol by Phanerochaete spp. Appl Environ Microbiol. 1990 Nov;56(11):3519–3526. doi: 10.1128/aem.56.11.3519-3526.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura F., Benezet H. J. Studies on the bioaccumulation and microbial degradation of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Environ Health Perspect. 1973 Sep;5:253–258. doi: 10.1289/ehp.7305253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszczynski A., Crawford R. L. Degradation of azo compounds by ligninase from Phanerochaete chrysosporium: involvement of veratryl alcohol. Biochem Biophys Res Commun. 1991 Aug 15;178(3):1056–1063. doi: 10.1016/0006-291x(91)90999-n. [DOI] [PubMed] [Google Scholar]
- Rose J. Q., Ramsey J. C., Wentzler T. H., Hummel R. A., Gehring P. J. The fate of 2,3,7,8-tetrachlorodibenzo-p-dioxin following single and repeated oral doses to the rat. Toxicol Appl Pharmacol. 1976 May;36(2):209–226. doi: 10.1016/0041-008x(76)90001-6. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Spain J. C., Gibson D. T. Pathway for Biodegradation of p-Nitrophenol in a Moraxella sp. Appl Environ Microbiol. 1991 Mar;57(3):812–819. doi: 10.1128/aem.57.3.812-819.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonon F., Odier E. Influence of Veratryl Alcohol and Hydrogen Peroxide on Ligninase Activity and Ligninase Production by Phanerochaete chrysosporium. Appl Environ Microbiol. 1988 Feb;54(2):466–472. doi: 10.1128/aem.54.2.466-472.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valli K., Brock B. J., Joshi D. K., Gold M. H. Degradation of 2,4-dinitrotoluene by the lignin-degrading fungus Phanerochaete chrysosporium. Appl Environ Microbiol. 1992 Jan;58(1):221–228. doi: 10.1128/aem.58.1.221-228.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valli K., Gold M. H. Degradation of 2,4-dichlorophenol by the lignin-degrading fungus Phanerochaete chrysosporium. J Bacteriol. 1991 Jan;173(1):345–352. doi: 10.1128/jb.173.1.345-352.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valli K., Wariishi H., Gold M. H. Oxidation of monomethoxylated aromatic compounds by lignin peroxidase: role of veratryl alcohol in lignin biodegradation. Biochemistry. 1990 Sep 18;29(37):8535–8539. doi: 10.1021/bi00489a005. [DOI] [PubMed] [Google Scholar]
- Wariishi H., Dunford H. B., MacDonald I. D., Gold M. H. Manganese peroxidase from the lignin-degrading basidiomycete Phanerochaete chrysosporium. Transient state kinetics and reaction mechanism. J Biol Chem. 1989 Feb 25;264(6):3335–3340. [PubMed] [Google Scholar]
- Wariishi H., Gold M. H. Lignin peroxidase compound III. Mechanism of formation and decomposition. J Biol Chem. 1990 Feb 5;265(4):2070–2077. [PubMed] [Google Scholar]
- Wariishi H., Valli K., Gold M. H. In vitro depolymerization of lignin by manganese peroxidase of Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1991 Apr 15;176(1):269–275. doi: 10.1016/0006-291x(91)90919-x. [DOI] [PubMed] [Google Scholar]
- Zemper E. D., Black S. H. Morphology of freeze-etched Treponema refringens (Nichols). Arch Microbiol. 1978 Jun 26;117(3):227–238. doi: 10.1007/BF00738540. [DOI] [PubMed] [Google Scholar]


