Abstract
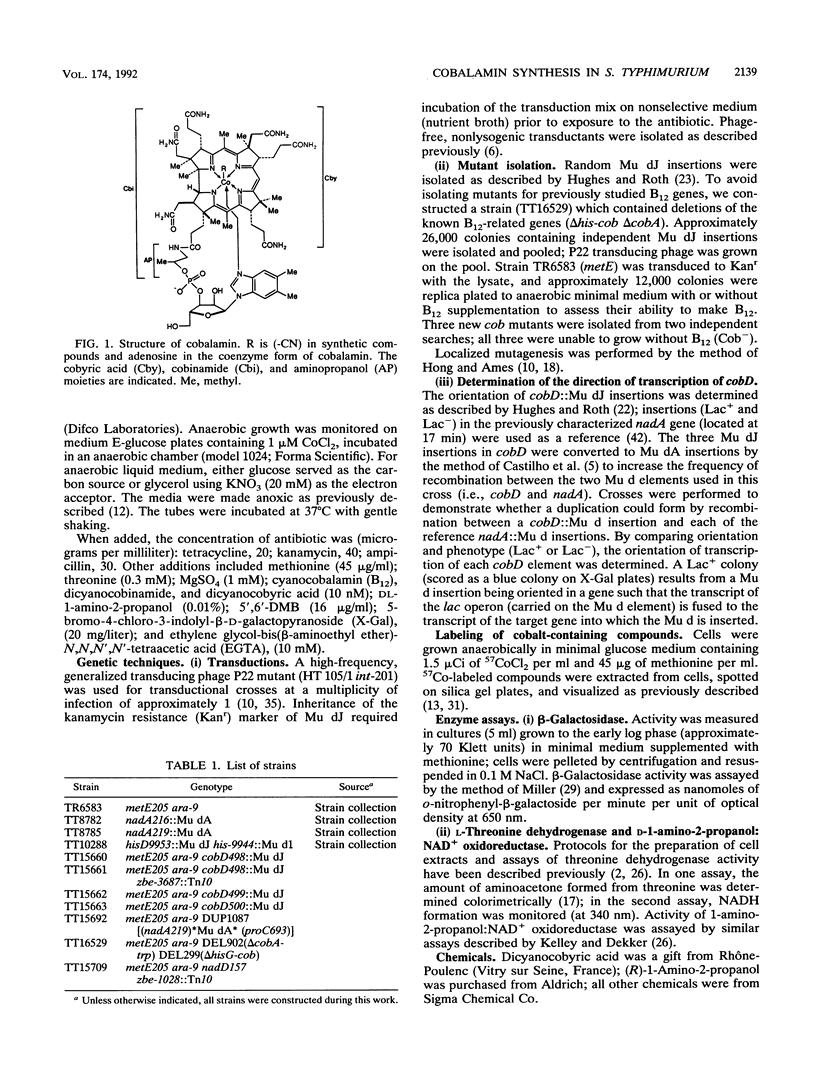
Salmonella typhimurium synthesizes cobalamin (vitamin B12) when grown under anaerobic conditions. All but one of the biosynthetic genes (cob) are located in a single operon which includes genes required for the production of cobinamide and dimethylbenzimidazole, as well as the genes needed to form cobalamin from these precursors. We isolated strains carrying mutations (cobD) which are unlinked to any of the previously described B12 biosynthetic genes. Mutations in cobD are recessive and map at minute 14 of the linkage map, far from the major cluster of B12 genes at minute 41. The cobD mutants appear to be defective in the synthesis of 1-amino-2-propanol, because they can synthesize B12 when this compound is provided exogenously. Labeling studies in other organisms have shown that aminopropanol, derived from threonine, is the precursor of the chain linking dimethylbenzimidazole to the corrinoid ring of B12. Previously, a three-step pathway has been proposed for the synthesis of aminopropanol from threonine, including two enzymatic steps and a spontaneous nonenzymatic decarboxylation. We assayed the two enzymatic steps of the hypothetical pathway; cobD mutants are not defective in either. Furthermore, mutants blocked in one step of the proposed pathway continue to make B12. We conclude that the aminopropanol for B12 synthesis is not made by this pathway. Expression of a lac operon fused to the cobD promoter is unaffected by vitamin B12 or oxygen, both of which are known to repress the main cob operon, suggesting that the cobD gene is not regulated.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson D. I., Roth J. R. Redox regulation of the genes for cobinamide biosynthesis in Salmonella typhimurium. J Bacteriol. 1989 Dec;171(12):6734–6739. doi: 10.1128/jb.171.12.6734-6739.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan S. A., Dekker E. E. Growth, enzyme levels, and some metabolic properties of an Escherichia coli mutant grown on L-threonine as the sole carbon source. J Bacteriol. 1983 Oct;156(1):273–280. doi: 10.1128/jb.156.1.273-280.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan S. A., Dekker E. E. L-threonine dehydrogenase. Purification and properties of the homogeneous enzyme from Escherichia coli K-12. J Biol Chem. 1981 Feb 25;256(4):1809–1815. [PubMed] [Google Scholar]
- Castilho B. A., Olfson P., Casadaban M. J. Plasmid insertion mutagenesis and lac gene fusion with mini-mu bacteriophage transposons. J Bacteriol. 1984 May;158(2):488–495. doi: 10.1128/jb.158.2.488-495.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R. K., Botstein D., Watanabe T., Ogata Y. Specialized transduction of tetracycline resistance by phage P22 in Salmonella typhimurium. II. Properties of a high-frequency-transducing lysate. Virology. 1972 Dec;50(3):883–898. doi: 10.1016/0042-6822(72)90442-4. [DOI] [PubMed] [Google Scholar]
- Chumley F. G., Menzel R., Roth J. R. Hfr formation directed by tn10. Genetics. 1979 Apr;91(4):639–655. doi: 10.1093/genetics/91.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouzet J., Cameron B., Cauchois L., Rigault S., Rouyez M. C., Blanche F., Thibaut D., Debussche L. Genetic and sequence analysis of an 8.7-kilobase Pseudomonas denitrificans fragment carrying eight genes involved in transformation of precorrin-2 to cobyrinic acid. J Bacteriol. 1990 Oct;172(10):5980–5990. doi: 10.1128/jb.172.10.5980-5990.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouzet J., Cauchois L., Blanche F., Debussche L., Thibaut D., Rouyez M. C., Rigault S., Mayaux J. F., Cameron B. Nucleotide sequence of a Pseudomonas denitrificans 5.4-kilobase DNA fragment containing five cob genes and identification of structural genes encoding S-adenosyl-L-methionine: uroporphyrinogen III methyltransferase and cobyrinic acid a,c-diamide synthase. J Bacteriol. 1990 Oct;172(10):5968–5979. doi: 10.1128/jb.172.10.5968-5979.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott T., Roth J. R. Characterization of Tn10d-Cam: a transposition-defective Tn10 specifying chloramphenicol resistance. Mol Gen Genet. 1988 Aug;213(2-3):332–338. doi: 10.1007/BF00339599. [DOI] [PubMed] [Google Scholar]
- Escalante-Semerena J. C., Roth J. R. Regulation of cobalamin biosynthetic operons in Salmonella typhimurium. J Bacteriol. 1987 May;169(5):2251–2258. doi: 10.1128/jb.169.5.2251-2258.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante-Semerena J. C., Suh S. J., Roth J. R. cobA function is required for both de novo cobalamin biosynthesis and assimilation of exogenous corrinoids in Salmonella typhimurium. J Bacteriol. 1990 Jan;172(1):273–280. doi: 10.1128/jb.172.1.273-280.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford S. H., Friedmann H. C. Vitamin B-12 biosynthesis. A model system for isopropanolamine formation by reaction between reduced corrinoid and threonine. Biochim Biophys Acta. 1977 Nov 7;500(1):217–222. doi: 10.1016/0304-4165(77)90062-9. [DOI] [PubMed] [Google Scholar]
- Ford S. H., Friedmann H. C. Vitamin B12 Biosynthesis: in vitro formation of cobinamide from cobyric acid and L-threonine. Arch Biochem Biophys. 1976 Jul;175(1):121–130. doi: 10.1016/0003-9861(76)90490-2. [DOI] [PubMed] [Google Scholar]
- GIBSON K. D., LAVER W. G., NEUBERGER A. Initial stages in the biosynthesis of porphyrins. 2. The formation of delta-aminolaevulic acid from glycine and succinyl-coenzyme A by particles from chicken erythrocytes. Biochem J. 1958 Sep;70(1):71–81. doi: 10.1042/bj0700071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J. S., Ames B. N. Localized mutagenesis of any specific small region of the bacterial chromosome. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3158–3162. doi: 10.1073/pnas.68.12.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K. T., Ladika D., Roth J. R., Olivera B. M. An indispensable gene for NAD biosynthesis in Salmonella typhimurium. J Bacteriol. 1983 Jul;155(1):213–221. doi: 10.1128/jb.155.1.213-221.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K. T., Roth J. R. Conditionally transposition-defective derivative of Mu d1(Amp Lac). J Bacteriol. 1984 Jul;159(1):130–137. doi: 10.1128/jb.159.1.130-137.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K. T., Roth J. R. Directed formation of deletions and duplications using Mud(Ap, lac). Genetics. 1985 Feb;109(2):263–282. doi: 10.1093/genetics/109.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K. T., Roth J. R. Transitory cis complementation: a method for providing transposition functions to defective transposons. Genetics. 1988 May;119(1):9–12. doi: 10.1093/genetics/119.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeter R. M., Roth J. R. Cobalamin (vitamin B12) biosynthetic genes of Salmonella typhimurium. J Bacteriol. 1987 Jul;169(7):3189–3198. doi: 10.1128/jb.169.7.3189-3198.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRASNA A. I., ROSENBLUM C., SPRINSON D. B. The conversion of L-threonine to the Dg-1-amino-2-propanol of vitamin B12. J Biol Chem. 1957 Apr;225(2):745–750. [PubMed] [Google Scholar]
- Kelley J. J., Dekker E. E. D-1-amino-2-propanol:NAD+ oxidoreductase. Purification and general properties of the large molecular form of the enzyme from Escherichia coli K12. J Biol Chem. 1984 Feb 25;259(4):2124–2129. [PubMed] [Google Scholar]
- NEUBERGER A., TAIT G. H. The enzymic conversion of threonine to aminoacetone. Biochim Biophys Acta. 1960 Jun 17;41:164–165. doi: 10.1016/0006-3002(60)90388-7. [DOI] [PubMed] [Google Scholar]
- Nexø E., Andersen J. Unsaturated and cobalamin saturated transcobalamin I and II in normal human plasma. Scand J Clin Lab Invest. 1977 Dec;37(8):723–728. doi: 10.3109/00365517709101856. [DOI] [PubMed] [Google Scholar]
- Potter R., Kapoor V., Newman E. B. Role of threonine dehydrogenase in Escherichia coli threonine degradation. J Bacteriol. 1977 Nov;132(2):385–391. doi: 10.1128/jb.132.2.385-391.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravnikar P. D., Somerville R. L. Genetic characterization of a highly efficient alternate pathway of serine biosynthesis in Escherichia coli. J Bacteriol. 1987 Jun;169(6):2611–2617. doi: 10.1128/jb.169.6.2611-2617.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravnikar P. D., Somerville R. L. Localization of the structural gene for threonine dehydrogenase in Escherichia coli. J Bacteriol. 1986 Oct;168(1):434–436. doi: 10.1128/jb.168.1.434-436.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieger H. A method for detection of phage mutants with altered transducing ability. Mol Gen Genet. 1971;110(4):378–381. doi: 10.1007/BF00438281. [DOI] [PubMed] [Google Scholar]
- Thibaut D., Blanche F., Debussche L., Leeper F. J., Battersby A. R. Biosynthesis of vitamin B12: structure of precorrin-6x octamethyl ester. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8800–8804. doi: 10.1073/pnas.87.22.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Warren M. J., Scott A. I. Tetrapyrrole assembly and modification into the ligands of biologically functional cofactors. Trends Biochem Sci. 1990 Dec;15(12):486–491. doi: 10.1016/0968-0004(90)90304-t. [DOI] [PubMed] [Google Scholar]
- Way J. C., Davis M. A., Morisato D., Roberts D. E., Kleckner N. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene. 1984 Dec;32(3):369–379. doi: 10.1016/0378-1119(84)90012-x. [DOI] [PubMed] [Google Scholar]
- Zhu N., Olivera B. M., Roth J. R. Genetic characterization of the pnuC gene, which encodes a component of the nicotinamide mononucleotide transport system in Salmonella typhimurium. J Bacteriol. 1989 Aug;171(8):4402–4409. doi: 10.1128/jb.171.8.4402-4409.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]