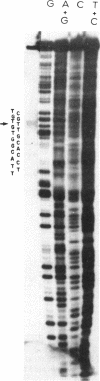
Abstract
The expression of the Kdp system for K+ uptake in Escherichia coli requires the products of two genes, kdpD and kdpE. These genes constitute an operon adjacent to the kdpABC operon that encodes the three membrane protein subunits of Kdp. Both operons are transcribed in the same direction and overlap; the kdpDE promoter is in kdpC, the last gene of the kdpABC operon. Transcription of the kdpDE operon is at a low level when Kdp is not expressed; transcription increases about 10-fold when kdpABC is turned on, indicating significant read-through of the kdpDE operon by transcripts beginning at the promoter of kdpABC operon. The proximal region of the kdpD gene is the site of most mutations that lead to constitutive expression of the kdpABC operon.
Full text
PDF


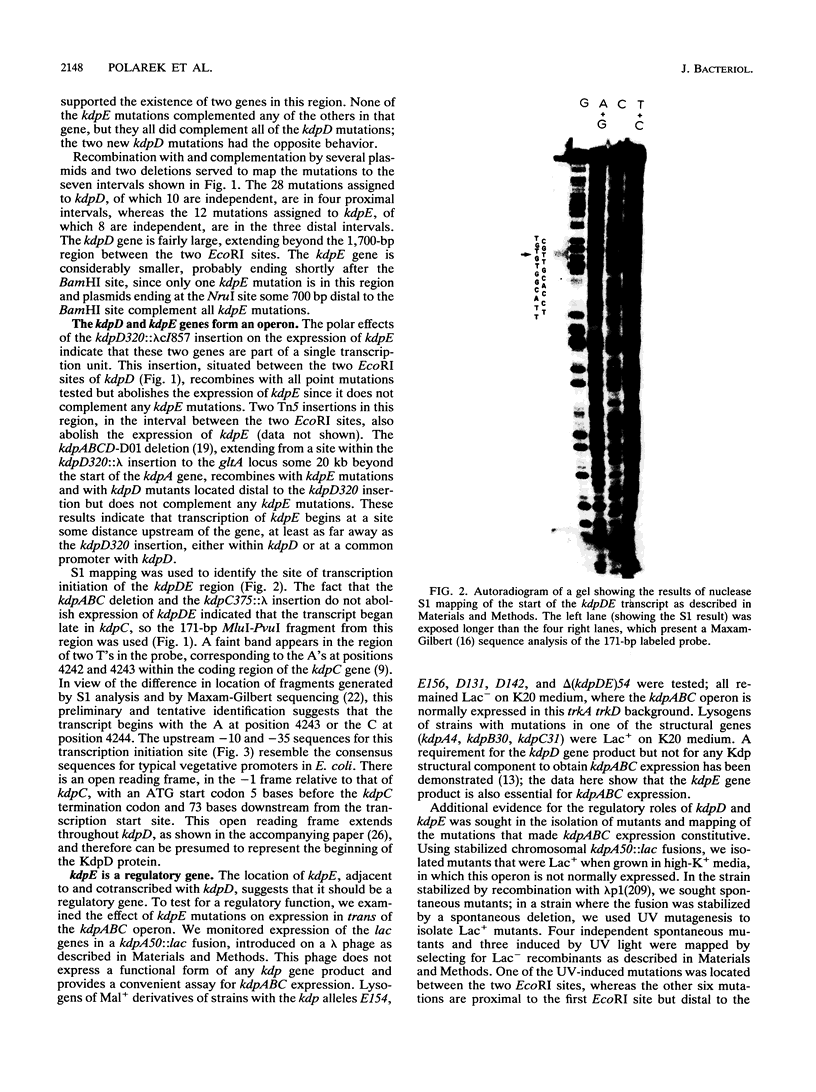



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKWITH J. RESTORATION OF OPERON ACTIVITY BY SUPPRESSORS. Biochim Biophys Acta. 1963 Sep 17;76:162–164. [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Bossemeyer D., Borchard A., Dosch D. C., Helmer G. C., Epstein W., Booth I. R., Bakker E. P. K+-transport protein TrkA of Escherichia coli is a peripheral membrane protein that requires other trk gene products for attachment to the cytoplasmic membrane. J Biol Chem. 1989 Oct 5;264(28):16403–16410. [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosch D. C., Helmer G. L., Sutton S. H., Salvacion F. F., Epstein W. Genetic analysis of potassium transport loci in Escherichia coli: evidence for three constitutive systems mediating uptake potassium. J Bacteriol. 1991 Jan;173(2):687–696. doi: 10.1128/jb.173.2.687-696.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Davies M. Potassium-dependant mutants of Escherichia coli K-12. J Bacteriol. 1970 Mar;101(3):836–843. doi: 10.1128/jb.101.3.836-843.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Kim B. S. Potassium transport loci in Escherichia coli K-12. J Bacteriol. 1971 Nov;108(2):639–644. doi: 10.1128/jb.108.2.639-644.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Walderhaug M. O., Polarek J. W., Hesse J. E., Dorus E., Daniel J. M. The bacterial Kdp K(+)-ATPase and its relation to other transport ATPases, such as the Na+/K(+)- and Ca2(+)-ATPases in higher organisms. Philos Trans R Soc Lond B Biol Sci. 1990 Jan 30;326(1236):479–487. doi: 10.1098/rstb.1990.0026. [DOI] [PubMed] [Google Scholar]
- Hesse J. E., Wieczorek L., Altendorf K., Reicin A. S., Dorus E., Epstein W. Sequence homology between two membrane transport ATPases, the Kdp-ATPase of Escherichia coli and the Ca2+-ATPase of sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4746–4750. doi: 10.1073/pnas.81.15.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins J. D. A new class of promoter mutations in the lactose operon of Escherichia coli. J Mol Biol. 1974 Aug 25;87(4):715–724. doi: 10.1016/0022-2836(74)90080-1. [DOI] [PubMed] [Google Scholar]
- Komeda Y., Iino T. Regulation of expression of the flagellin gene (hag) in Escherichia coli K-12: analysis of hag-lac gene fusions. J Bacteriol. 1979 Sep;139(3):721–729. doi: 10.1128/jb.139.3.721-729.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwano M., Schlessinger D., Morse D. E. Loss of dispensable endonuclease activity in relief of polarity by suA. Nat New Biol. 1971 Jun 16;231(24):214–217. doi: 10.1038/newbio231214a0. [DOI] [PubMed] [Google Scholar]
- Laimins L. A., Rhoads D. B., Altendorf K., Epstein W. Identification of the structural proteins of an ATP-driven potassium transport system in Escherichia coli. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3216–3219. doi: 10.1073/pnas.75.7.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins L. A., Rhoads D. B., Epstein W. Osmotic control of kdp operon expression in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Jan;78(1):464–468. doi: 10.1073/pnas.78.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Polarek J. W., Walderhaug M. O., Epstein W. Genetics of Kdp, the K+-transport ATPase of Escherichia coli. Methods Enzymol. 1988;157:655–667. doi: 10.1016/0076-6879(88)57113-6. [DOI] [PubMed] [Google Scholar]
- Rhoads D. B., Laimins L., Epstein W. Functional organization of the kdp genes of Escherichia coli K-12. J Bacteriol. 1978 Aug;135(2):445–452. doi: 10.1128/jb.135.2.445-452.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads D. B., Waters F. B., Epstein W. Cation transport in Escherichia coli. VIII. Potassium transport mutants. J Gen Physiol. 1976 Mar;67(3):325–341. doi: 10.1085/jgp.67.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salser W., Gesteland R. F., Bolle A. In vitro synthesis of bacteriophage lysozyme. Nature. 1967 Aug 5;215(5101):588–591. doi: 10.1038/215588a0. [DOI] [PubMed] [Google Scholar]
- Sollner-Webb B., Reeder R. H. The nucleotide sequence of the initiation and termination sites for ribosomal RNA transcription in X. laevis. Cell. 1979 Oct;18(2):485–499. doi: 10.1016/0092-8674(79)90066-7. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Ninfa A. J., Stock A. M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno-Nishio S., Mango S., Reitzer L. J., Magasanik B. Identification and regulation of the glnL operator-promoter of the complex glnALG operon of Escherichia coli. J Bacteriol. 1984 Oct;160(1):379–384. doi: 10.1128/jb.160.1.379-384.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walderhaug M. O., Polarek J. W., Voelkner P., Daniel J. M., Hesse J. E., Altendorf K., Epstein W. KdpD and KdpE, proteins that control expression of the kdpABC operon, are members of the two-component sensor-effector class of regulators. J Bacteriol. 1992 Apr;174(7):2152–2159. doi: 10.1128/jb.174.7.2152-2159.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]