Abstract
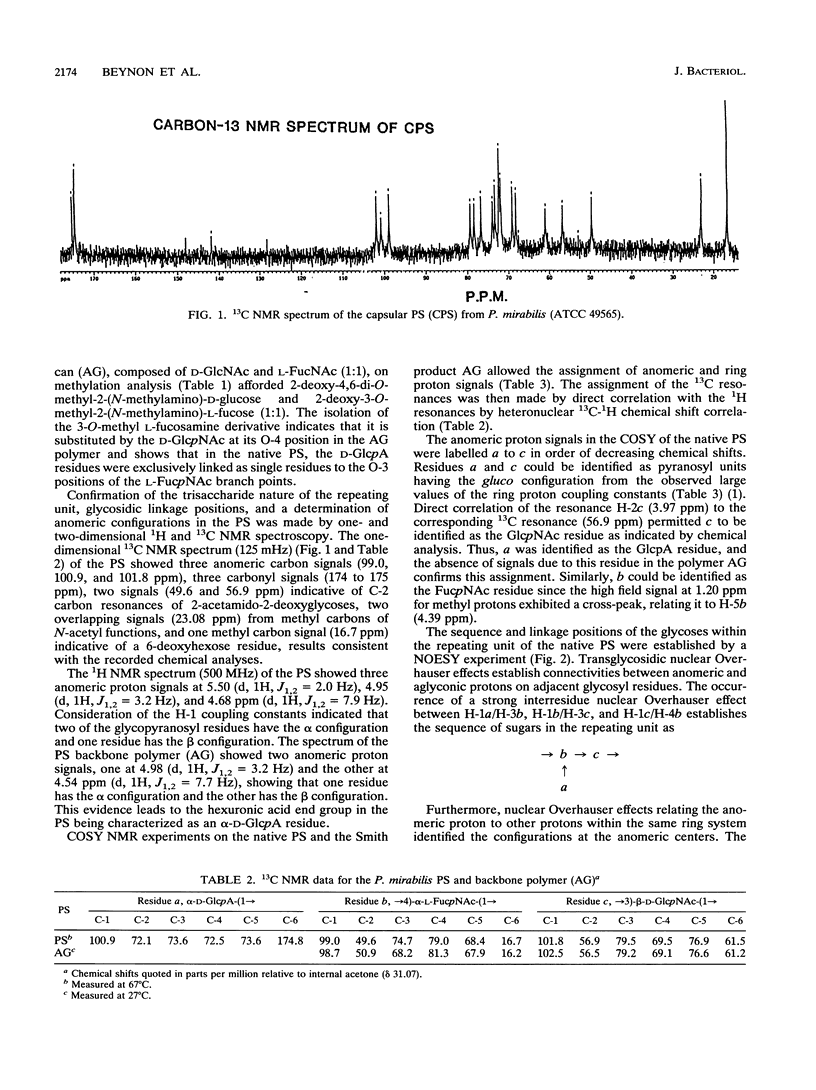
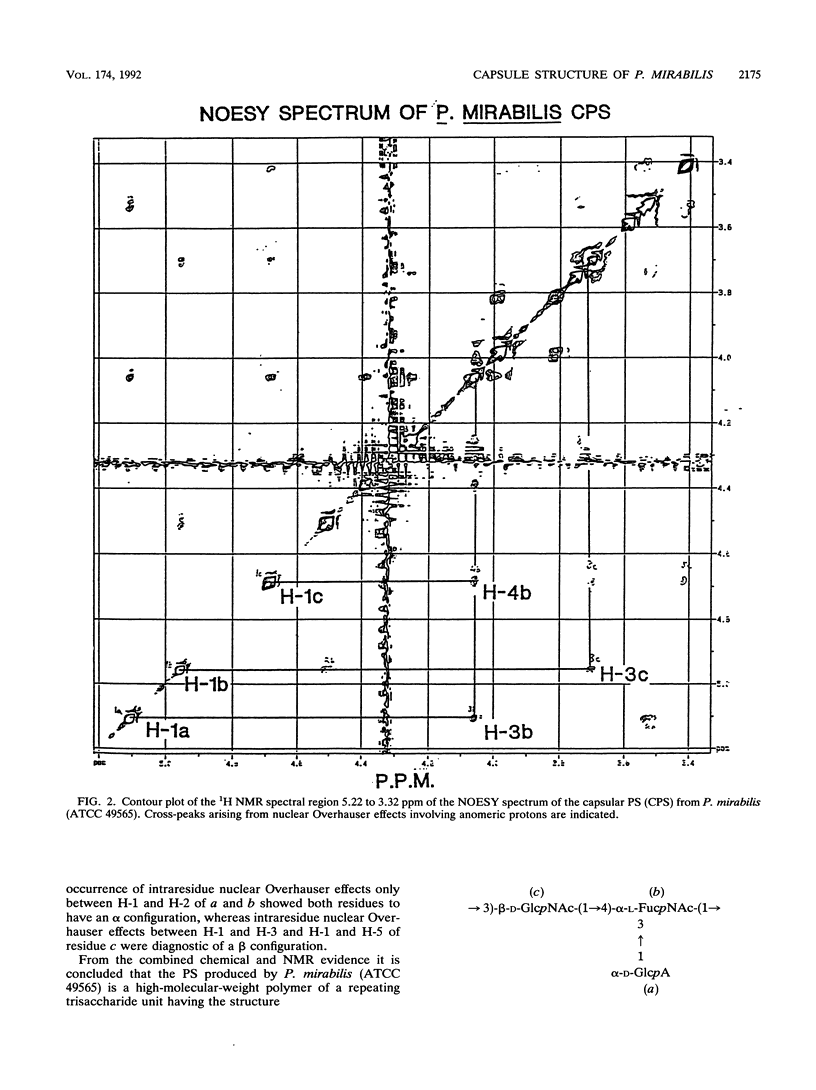
Proteus mirabilis 2573 (ATCC 49565) produces an acidic capsular polysaccharide which was shown from glycose analysis, carboxyl reduction, methylation, periodate oxidation, and the application of one dimensional and two-dimensional high-resolution nuclear magnetic resonance techniques to be a high-molecular-weight polymer of branched trisaccharide units composed of 2-acetamido-2-deoxy-D-glucose (N-acetyl-D-glucosamine), 2-acetamido-2,6-dideoxy-L-galactose (N-acetyl-L-fucosamine), and D-glucuronic acid, having the structure: [formula: see text] P. mirabilis 2573 also produces an O:6 serotype lipopolysaccharide in which the O-chain component has the same structure as the homologous capsular polysaccharide. This is the first report of a defined capsular polysaccharide in this bacterial genus.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beynon L. M., Perry M. B., Richards J. C. Structure of the capsular polysaccharide from Actinobacillus pleuropneumoniae serotype 7. Carbohydr Res. 1991 Jan 15;209:211–223. doi: 10.1016/0008-6215(91)80158-j. [DOI] [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Gatt R., Berman E. R. A rapid procedure for the estimation of amino sugars on a micro scale. Anal Biochem. 1966 Apr;15(1):167–171. doi: 10.1016/0003-2697(66)90262-4. [DOI] [PubMed] [Google Scholar]
- Goldman R. C., White D., Orskov F., Orskov I., Rick P. D., Lewis M. S., Bhattacharjee A. K., Leive L. A surface polysaccharide of Escherichia coli O111 contains O-antigen and inhibits agglutination of cells by O-antiserum. J Bacteriol. 1982 Sep;151(3):1210–1221. doi: 10.1128/jb.151.3.1210-1221.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham L. L., Harris R., Villiger W., Beveridge T. J. Freeze-substitution of gram-negative eubacteria: general cell morphology and envelope profiles. J Bacteriol. 1991 Mar;173(5):1623–1633. doi: 10.1128/jb.173.5.1623-1633.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith D. P., Musher D. M., Itin C. Urease. The primary cause of infection-induced urinary stones. Invest Urol. 1976 Mar;13(5):346–350. [PubMed] [Google Scholar]
- Johnson K. G., Perry M. B. Improved techniques for the preparation of bacterial lipopolysaccharides. Can J Microbiol. 1976 Jan;22(1):29–34. doi: 10.1139/m76-004. [DOI] [PubMed] [Google Scholar]
- Kumar A., Ernst R. R., Wüthrich K. A two-dimensional nuclear Overhauser enhancement (2D NOE) experiment for the elucidation of complete proton-proton cross-relaxation networks in biological macromolecules. Biochem Biophys Res Commun. 1980 Jul 16;95(1):1–6. doi: 10.1016/0006-291x(80)90695-6. [DOI] [PubMed] [Google Scholar]
- Mackie E. B., Brown K. N., Lam J., Costerton J. W. Morphological stabilization of capsules of group B streptococci, types Ia, Ib, II, and III, with specific antibody. J Bacteriol. 1979 May;138(2):609–617. doi: 10.1128/jb.138.2.609-617.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean R. J., Lawrence J. R., Korber D. R., Caldwell D. E. Proteus mirabilis biofilm protection against struvite crystal dissolution and its implications in struvite urolithiasis. J Urol. 1991 Oct;146(4):1138–1142. doi: 10.1016/s0022-5347(17)38026-6. [DOI] [PubMed] [Google Scholar]
- McLean R. J., Nickel J. C., Cheng K. J., Costerton J. W. The ecology and pathogenicity of urease-producing bacteria in the urinary tract. Crit Rev Microbiol. 1988;16(1):37–79. doi: 10.3109/10408418809104467. [DOI] [PubMed] [Google Scholar]
- McLean R. J., Nickel J. C., Noakes V. C., Costerton J. W. An in vitro ultrastructural study of infectious kidney stone genesis. Infect Immun. 1985 Sep;49(3):805–811. doi: 10.1128/iai.49.3.805-811.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner J. L., Hennessy J. N. Separate O-grouping schemes for serotyping clinical isolates of Proteus vulgaris and Proteus mirabilis. J Clin Microbiol. 1980 Sep;12(3):304–309. doi: 10.1128/jcm.12.3.304-309.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl S. J., Stewart K. R., Williams F. D. Extracellular slime associated with Proteus mirabilis during swarming. J Bacteriol. 1983 May;154(2):930–937. doi: 10.1128/jb.154.2.930-937.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]


