Abstract
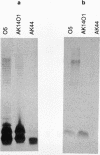
Serotypes O2, O5, and O16 of Pseudomonas aeruginosa are chemically related, and the O antigens of their lipopolysaccharides share a similar trisaccharide repeat backbone structure. Serotype-specific monoclonal antibodies (MAbs) MF71-3, MF15-4, and MF47-4 against the O2, O5, and O16 serotypes, respectively, were isolated. MAb 18-19, which is cross-reactive with all strains of this chemically related serogroup, was also produced. When column chromatography or sodium dodecyl sulfate-polyacrylamide gel electrophoresis-separated lipopolysaccharide (LPS) samples from each of the serotypes were probed with the MAbs in Western immunoblots, each of the serotype-specific MAbs interacted only with high-molecular-weight bands of the homologous LPS, with a minimum O-antigen chain length of at least 6 to 10 repeats. In contrast, cross-reactive MAb 18-19 was shown to interact in Western immunoblots with the entire LPS banding pattern except the fastest-running band, which lacks O antigen. Chemical modification of P. aeruginosa LPS by alkali treatment and carboxyl reduction abolished reactions between LPS and MAb 18-19, while reactions of modified LPS with serotype-specific MAbs were not affected. Therefore, cross-reactive MAb 18-19 likely recognizes the chemical backbone structure of the O repeat that is common to all three serotypes of the O2-O5-O16 group, while the O-specific MAbs appeared to recognize LPS epitopes that could be presented when 6 to 10 or more O-antigen repeat units are present on the LPS molecule. Thus, the O-specific LPS epitopes likely involve unique chemical structures, glycosidic linkages, and some order of folding of the O side chains.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry D., Kropinski A. M. Effect of lipopolysaccharide mutations and temperature on plasmid transformation efficiency in Pseudomonas aeruginosa. Can J Microbiol. 1986 May;32(5):436–438. doi: 10.1139/m86-082. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Dubray G., Bezard G. A highly sensitive periodic acid-silver stain for 1,2-diol groups of glycoproteins and polysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 15;119(2):325–329. doi: 10.1016/0003-2697(82)90593-0. [DOI] [PubMed] [Google Scholar]
- Dupont C., Clarke A. J. Dependence of lysozyme-catalysed solubilization of Proteus mirabilis peptidoglycan on the extent of O-acetylation. Eur J Biochem. 1991 Feb 14;195(3):763–769. doi: 10.1111/j.1432-1033.1991.tb15764.x. [DOI] [PubMed] [Google Scholar]
- Finne J., Bitter-Suermann D., Goridis C., Finne U. An IgG monoclonal antibody to group B meningococci cross-reacts with developmentally regulated polysialic acid units of glycoproteins in neural and extraneural tissues. J Immunol. 1987 Jun 15;138(12):4402–4407. [PubMed] [Google Scholar]
- Gatt R., Berman E. R. A rapid procedure for the estimation of amino sugars on a micro scale. Anal Biochem. 1966 Apr;15(1):167–171. doi: 10.1016/0003-2697(66)90262-4. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Mutharia L. M., Chan L., Darveau R. P., Speert D. P., Pier G. B. Pseudomonas aeruginosa isolates from patients with cystic fibrosis: a class of serum-sensitive, nontypable strains deficient in lipopolysaccharide O side chains. Infect Immun. 1983 Oct;42(1):170–177. doi: 10.1128/iai.42.1.170-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen W., Yourassowsky E. Recherche de glycosidases chez les Pseudomonas du groupe fluorescent: relation entre sérogroupe et activités glycosidasiques chez P. aeruginosa. Ann Microbiol (Paris) 1983 Nov-Dec;134B(3):411–419. [PubMed] [Google Scholar]
- Häyrinen J., Bitter-Suermann D., Finne J. Interaction of meningococcal group B monoclonal antibody and its Fab fragment with alpha 2-8-linked sialic acid polymers: requirement of a long oligosaccharide segment for binding. Mol Immunol. 1989 Jun;26(6):523–529. doi: 10.1016/0161-5890(89)90003-5. [DOI] [PubMed] [Google Scholar]
- Jennings H. J., Roy R., Gamian A. Induction of meningococcal group B polysaccharide-specific IgG antibodies in mice by using an N-propionylated B polysaccharide-tetanus toxoid conjugate vaccine. J Immunol. 1986 Sep 1;137(5):1708–1713. [PubMed] [Google Scholar]
- Knirel YuA, Vinogradov E. V., Kocharova N. A., Paramonov N. A., Kochetkov N. K., Dmitriev B. A., Stanislavsky E. S., Lányi B. The structure of O-specific polysaccharides and serological classification of Pseudomonas aeruginosa (a review). Acta Microbiol Hung. 1988;35(1):3–24. [PubMed] [Google Scholar]
- Knirel Y. A., Vinogradov E. V., Shashkov A. S., Dmitriev B. A., Kochetkov N. K., Stanislavsky E. S., Mashilova G. M. Somatic antigens of Pseudomonas aeruginosa. The structure of O-specific polysaccharide chains of P. aeruginosa O:3a, b and O:3a, d lipopolysaccharides. Eur J Biochem. 1982 Nov;128(1):81–90. [PubMed] [Google Scholar]
- Kropinski A. M., Chan L. C., Milazzo F. H. The extraction and analysis of lipopolysaccharides from Pseudomonas aeruginosa strain PAO, and three rough mutants. Can J Microbiol. 1979 Mar;25(3):390–398. doi: 10.1139/m79-060. [DOI] [PubMed] [Google Scholar]
- Kropinski A. M., Kuzio J., Angus B. L., Hancock R. E. Chemical and chromatographic analysis of lipopolysaccharide from an antibiotic-supersusceptible mutant of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1982 Feb;21(2):310–319. doi: 10.1128/aac.21.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzio J., Kropinski A. M. O-antigen conversion in Pseudomonas aeruginosa PAO1 by bacteriophage D3. J Bacteriol. 1983 Jul;155(1):203–212. doi: 10.1128/jb.155.1.203-212.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J. S., MacDonald L. A., Lam M. Y., Duchesne L. G., Southam G. G. Production and characterization of monoclonal antibodies against serotype strains of Pseudomonas aeruginosa. Infect Immun. 1987 May;55(5):1051–1057. doi: 10.1128/iai.55.5.1051-1057.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J. S., MacDonald L. A., Lam M. Y. Production of monoclonal antibodies against serotype strains of Pseudomonas aeruginosa. Infect Immun. 1987 Nov;55(11):2854–2856. doi: 10.1128/iai.55.11.2854-2856.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam M. Y., McGroarty E. J., Kropinski A. M., MacDonald L. A., Pedersen S. S., Høiby N., Lam J. S. Occurrence of a common lipopolysaccharide antigen in standard and clinical strains of Pseudomonas aeruginosa. J Clin Microbiol. 1989 May;27(5):962–967. doi: 10.1128/jcm.27.5.962-967.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P. V., Wang S. Three new major somatic antigens of Pseudomonas aeruginosa. J Clin Microbiol. 1990 May;28(5):922–925. doi: 10.1128/jcm.28.5.922-925.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeniyi B., Lam J. S., Høiby N., Rosdahl V. T. A comparison of the efficiency in serotyping of Pseudomonas aeruginosa from cystic fibrosis patients using monoclonal and polyclonal antibodies. APMIS. 1989 Jul;97(7):631–636. doi: 10.1111/j.1699-0463.1989.tb00454.x. [DOI] [PubMed] [Google Scholar]
- Rivera M., Bryan L. E., Hancock R. E., McGroarty E. J. Heterogeneity of lipopolysaccharides from Pseudomonas aeruginosa: analysis of lipopolysaccharide chain length. J Bacteriol. 1988 Feb;170(2):512–521. doi: 10.1128/jb.170.2.512-521.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. L., Conrad H. E. Stoichiometric depolymerization of polyuronides and glycosaminoglycuronans to monosaccharides following reduction of their carbodiimide-activated carboxyl groups. Biochemistry. 1972 Apr 11;11(8):1383–1388. doi: 10.1021/bi00758a009. [DOI] [PubMed] [Google Scholar]
- Vale T. A., Gaston M. A., Pitt T. L. Subdivision of O serotypes of Pseudomonas aeruginosa with monoclonal antibodies. J Clin Microbiol. 1988 Sep;26(9):1779–1782. doi: 10.1128/jcm.26.9.1779-1782.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]