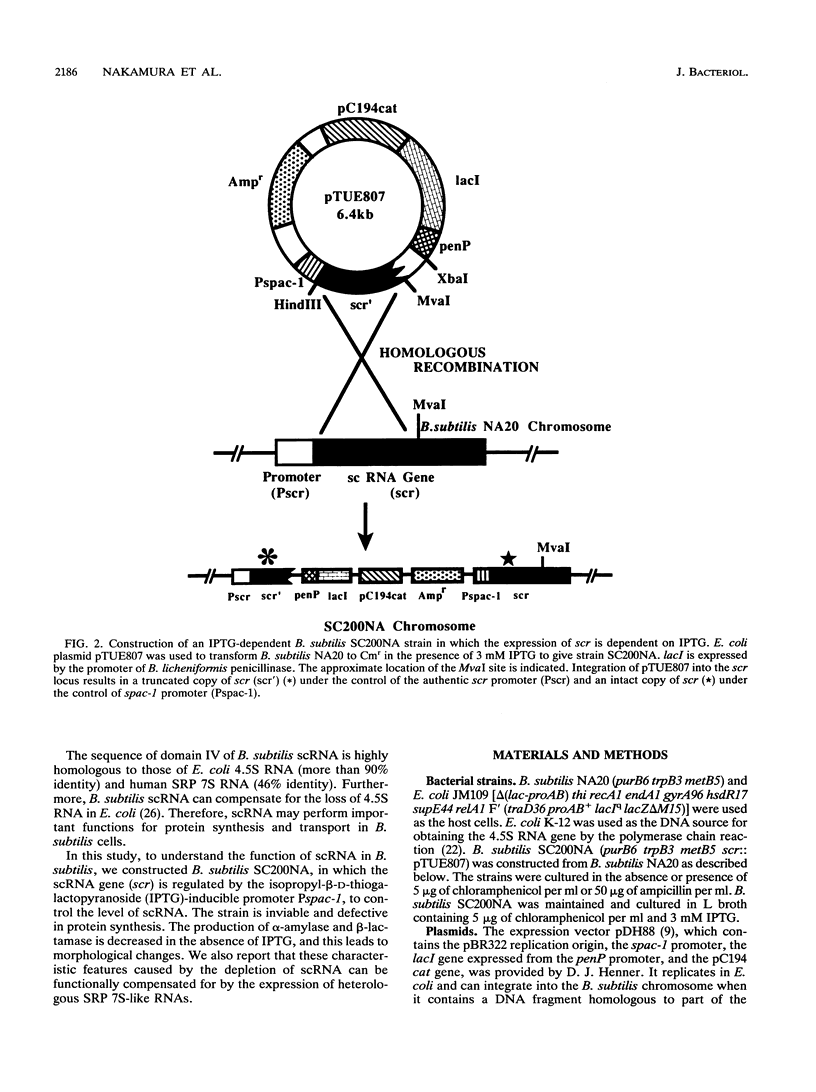
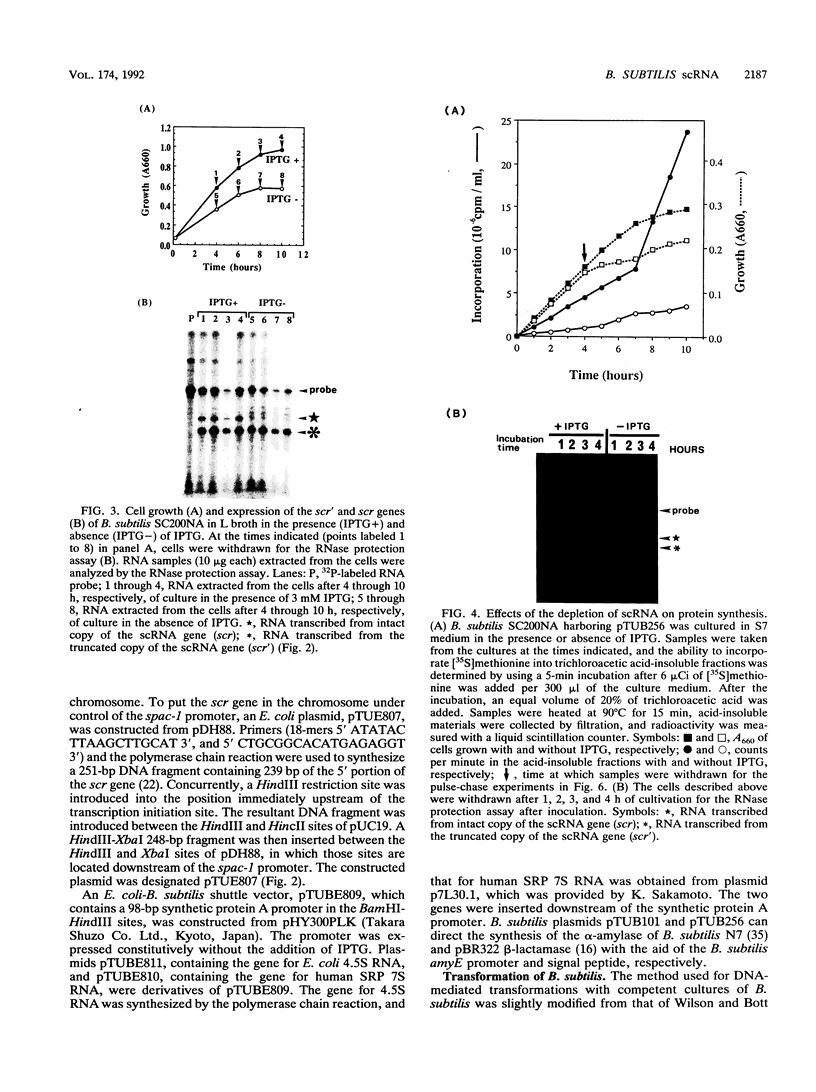
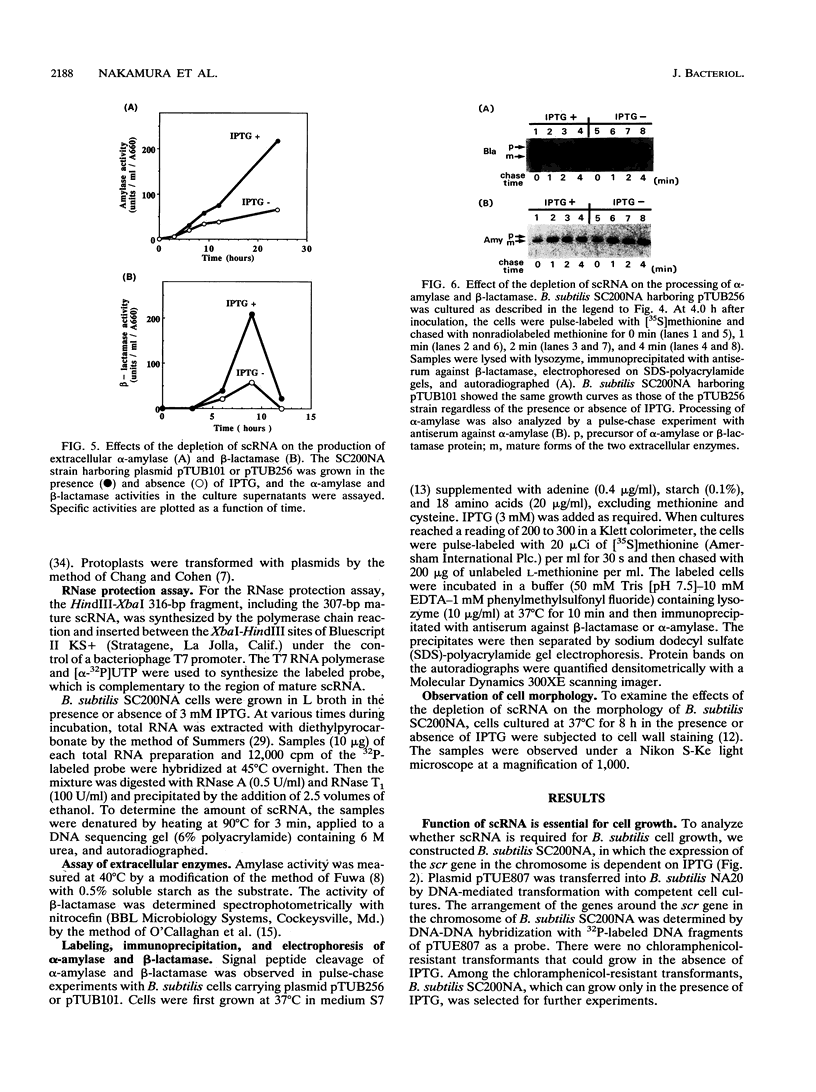
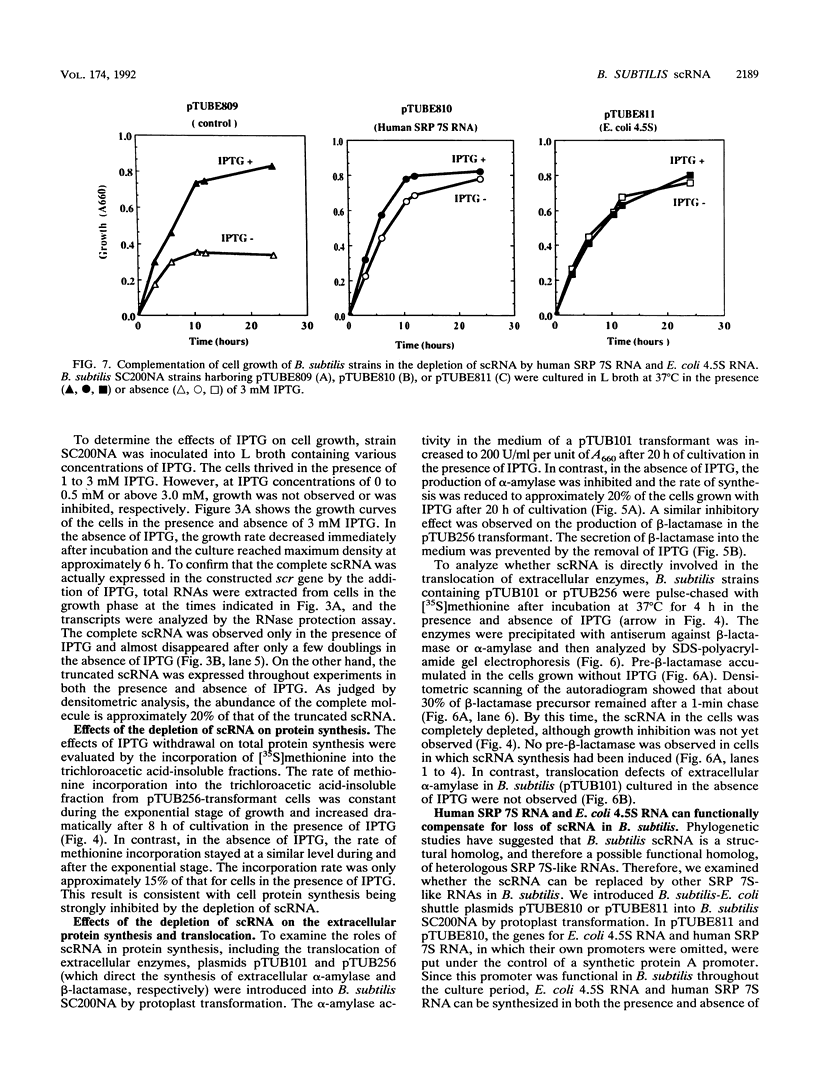
Abstract
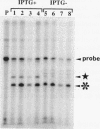
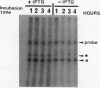
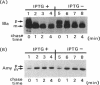
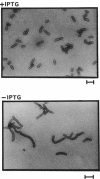
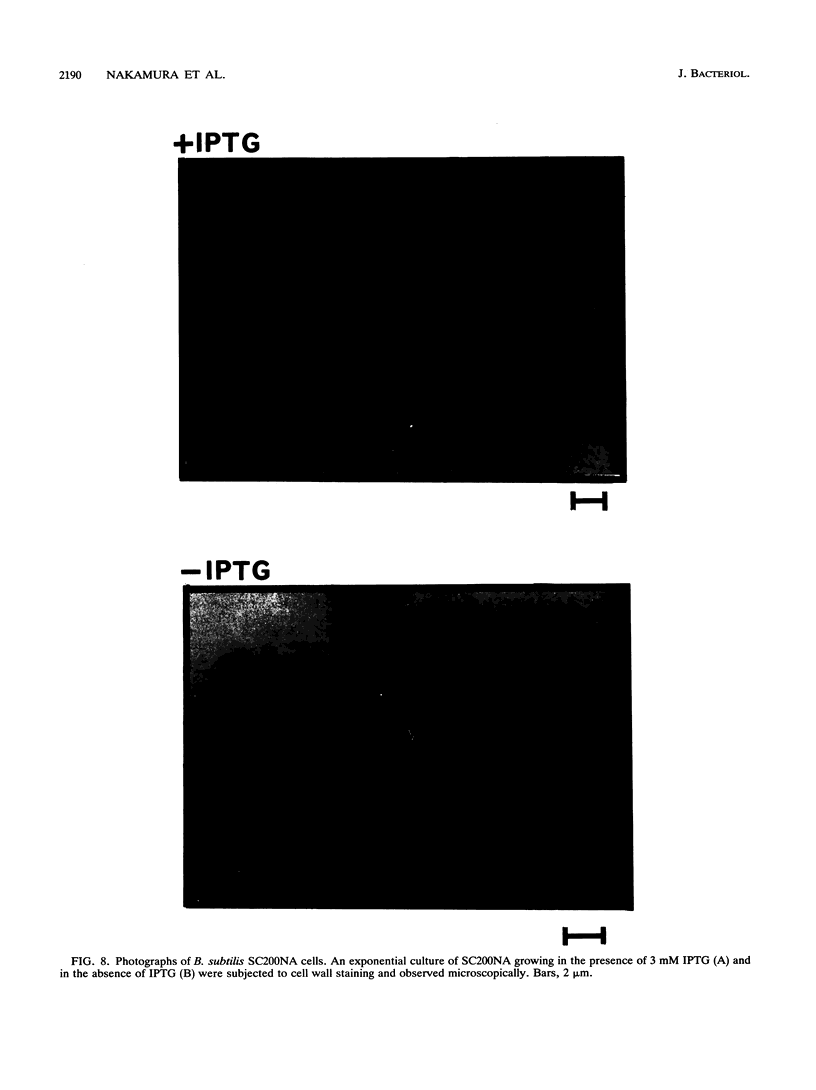
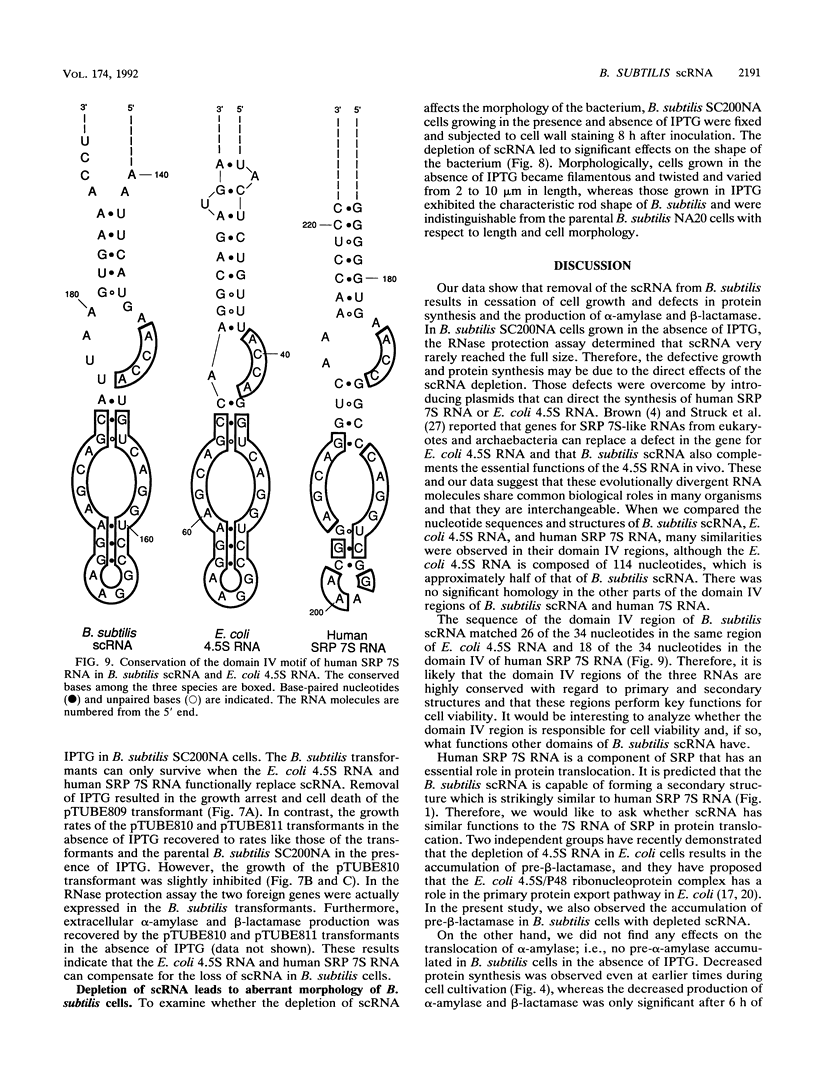
Small cytoplasmic RNA (scRNA; 271 nucleotides) is an abundant and stable RNA of the gram-positive bacterium Bacillus subtilis. To investigate the function of scRNA in B. subtilis cells, we developed a strain that is dependent on isopropyl-beta-D-thiogalactopyranoside for scRNA synthesis by fusing the chromosomal scr locus with the spac-1 promoter by homologous recombination. Depletion of the inducer leads to a loss of scRNA synthesis, defects in protein synthesis and production of alpha-amylase and beta-lactamase, and eventual cell death. The loss of the scRNA gene in B. subtilis can be complemented by the introduction of human signal recognition particle 7S RNA, which is considered to be involved in protein transport, or Escherichia coli 4.5S RNA. These results provide further evidence for a functional relationship between B. subtilis scRNA, human signal recognition particle 7S RNA, and E. coli 4.5S RNA.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourgaize D. B., Fournier M. J. Initiation of translation is impaired in E. coli cells deficient in 4.5S RNA. Nature. 1987 Jan 15;325(6101):281–284. doi: 10.1038/325281a0. [DOI] [PubMed] [Google Scholar]
- Bourgaize D. B., Phillips T. A., VanBogelen R. A., Jones P. G., Neidhardt F. C., Fournier M. J. Loss of 4.5S RNA induces the heat shock response and lambda prophage in Escherichia coli. J Bacteriol. 1990 Feb;172(2):1151–1154. doi: 10.1128/jb.172.2.1151-1154.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennwald P., Liao X., Holm K., Porter G., Wise J. A. Identification of an essential Schizosaccharomyces pombe RNA homologous to the 7SL component of signal recognition particle. Mol Cell Biol. 1988 Apr;8(4):1580–1590. doi: 10.1128/mcb.8.4.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S., Fournier M. J. The 4.5 S RNA gene of Escherichia coli is essential for cell growth. J Mol Biol. 1984 Sep 25;178(3):533–550. doi: 10.1016/0022-2836(84)90237-7. [DOI] [PubMed] [Google Scholar]
- Brown S. Genes for 7S RNAs can replace the gene for 4.5S RNA in growth of Escherichia coli. J Bacteriol. 1991 Mar;173(5):1835–1837. doi: 10.1128/jb.173.5.1835-1837.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos N., Palau J., Zwieb C. Diversity of 7 SL RNA from the signal recognition particle of maize endosperm. Nucleic Acids Res. 1989 Feb 25;17(4):1573–1588. doi: 10.1093/nar/17.4.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S., Cohen S. N. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet. 1979 Jan 5;168(1):111–115. doi: 10.1007/BF00267940. [DOI] [PubMed] [Google Scholar]
- Henner D. J. Inducible expression of regulatory genes in Bacillus subtilis. Methods Enzymol. 1990;185:223–228. doi: 10.1016/0076-6879(90)85022-g. [DOI] [PubMed] [Google Scholar]
- Hortsch M., Meyer D. I. Transfer of secretory proteins through the membrane of the endoplasmic reticulum. Int Rev Cytol. 1986;102:215–242. doi: 10.1016/s0074-7696(08)61276-0. [DOI] [PubMed] [Google Scholar]
- Kaine B. P. Structure of the archaebacterial 7S RNA molecule. Mol Gen Genet. 1990 May;221(3):315–321. doi: 10.1007/BF00259394. [DOI] [PubMed] [Google Scholar]
- Nagarajan V. System for secretion of heterologous proteins in Bacillus subtilis. Methods Enzymol. 1990;185:214–223. doi: 10.1016/0076-6879(90)85021-f. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Nakamura A., Takamatsu H., Yoshikawa H., Yamane K. Cloning and characterization of a Bacillus subtilis gene homologous to E. coli secY. J Biochem. 1990 Apr;107(4):603–607. doi: 10.1093/oxfordjournals.jbchem.a123093. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmura K., Shiroza T., Nakamura K., Nakayama A., Yamane K., Yoda K., Yamasaki M., Tamura G. A Bacillus subtilis secretion vector system derived from the B. subtilis alpha-amylase promoter and signal sequence region, and secretion of Escherichia coli beta-lactamase by the vector system. J Biochem. 1984 Jan;95(1):87–93. doi: 10.1093/oxfordjournals.jbchem.a134607. [DOI] [PubMed] [Google Scholar]
- Poritz M. A., Bernstein H. D., Strub K., Zopf D., Wilhelm H., Walter P. An E. coli ribonucleoprotein containing 4.5S RNA resembles mammalian signal recognition particle. Science. 1990 Nov 23;250(4984):1111–1117. doi: 10.1126/science.1701272. [DOI] [PubMed] [Google Scholar]
- Poritz M. A., Siegel V., Hansen W., Walter P. Small ribonucleoproteins in Schizosaccharomyces pombe and Yarrowia lipolytica homologous to signal recognition particle. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4315–4319. doi: 10.1073/pnas.85.12.4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poritz M. A., Strub K., Walter P. Human SRP RNA and E. coli 4.5S RNA contain a highly homologous structural domain. Cell. 1988 Oct 7;55(1):4–6. doi: 10.1016/0092-8674(88)90003-7. [DOI] [PubMed] [Google Scholar]
- Ribes V., Römisch K., Giner A., Dobberstein B., Tollervey D. E. coli 4.5S RNA is part of a ribonucleoprotein particle that has properties related to signal recognition particle. Cell. 1990 Nov 2;63(3):591–600. doi: 10.1016/0092-8674(90)90454-m. [DOI] [PubMed] [Google Scholar]
- Sadaie Y., Takamatsu H., Nakamura K., Yamane K. Sequencing reveals similarity of the wild-type div+ gene of Bacillus subtilis to the Escherichia coli secA gene. Gene. 1991 Feb 1;98(1):101–105. doi: 10.1016/0378-1119(91)90110-w. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanz P., Meyer D. I. Signal recognition particle (SRP) stabilizes the translocation-competent conformation of pre-secretory proteins. EMBO J. 1988 Nov;7(11):3553–3557. doi: 10.1002/j.1460-2075.1988.tb03232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz P. J., Beckwith J. Genetic analysis of protein export in Escherichia coli. Annu Rev Genet. 1990;24:215–248. doi: 10.1146/annurev.ge.24.120190.001243. [DOI] [PubMed] [Google Scholar]
- Struck J. C., Hartmann R. K., Toschka H. Y., Erdmann V. A. Transcription and processing of Bacillus subtilis small cytoplasmic RNA. Mol Gen Genet. 1989 Feb;215(3):478–482. doi: 10.1007/BF00427046. [DOI] [PubMed] [Google Scholar]
- Struck J. C., Lempicki R. A., Toschka H. Y., Erdmann V. A., Fournier M. J. Escherichia coli 4.5S RNA gene function can be complemented by heterologous bacterial RNA genes. J Bacteriol. 1990 Mar;172(3):1284–1288. doi: 10.1128/jb.172.3.1284-1288.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struck J. C., Vogel D. W., Ulbrich N., Erdmann V. A. The Bacillus subtilis scRNA is related to the 4.5S RNA from Escherichia coli. Nucleic Acids Res. 1988 Mar 25;16(6):2719–2719. doi: 10.1093/nar/16.6.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh J. W., Boylan S. A., Thomas S. M., Dolan K. M., Oliver D. B., Price C. W. Isolation of a secY homologue from Bacillus subtilis: evidence for a common protein export pathway in eubacteria. Mol Microbiol. 1990 Feb;4(2):305–314. doi: 10.1111/j.1365-2958.1990.tb00597.x. [DOI] [PubMed] [Google Scholar]
- Summers W. C. A simple method for extraction of RNA from E. coli utilizing diethyl pyrocarbonate. Anal Biochem. 1970 Feb;33(2):459–463. doi: 10.1016/0003-2697(70)90316-7. [DOI] [PubMed] [Google Scholar]
- Ullu E., Weiner A. M. Human genes and pseudogenes for the 7SL RNA component of signal recognition particle. EMBO J. 1984 Dec 20;3(13):3303–3310. doi: 10.1002/j.1460-2075.1984.tb02294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Purification of a membrane-associated protein complex required for protein translocation across the endoplasmic reticulum. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7112–7116. doi: 10.1073/pnas.77.12.7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature. 1982 Oct 21;299(5885):691–698. doi: 10.1038/299691a0. [DOI] [PubMed] [Google Scholar]
- Walter P., Lingappa V. R. Mechanism of protein translocation across the endoplasmic reticulum membrane. Annu Rev Cell Biol. 1986;2:499–516. doi: 10.1146/annurev.cb.02.110186.002435. [DOI] [PubMed] [Google Scholar]
- Wilson G. A., Bott K. F. Nutritional factors influencing the development of competence in the Bacillus subtilis transformation system. J Bacteriol. 1968 Apr;95(4):1439–1449. doi: 10.1128/jb.95.4.1439-1449.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane K., Hirata Y., Furusato T., Yamazaki H., Nakayama A. Changes in the properties and molecular weights of Bacillus subtilis M-type and N-type alpha-amylases resulting from a spontaneous deletion. J Biochem. 1984 Dec;96(6):1849–1858. doi: 10.1093/oxfordjournals.jbchem.a135019. [DOI] [PubMed] [Google Scholar]