Abstract
The expression of short- and long-term synaptic plasticity varies strongly across pathways in the central nervous system. Differences in the properties of transmitter release may underlie some of this variability. Here we compared the short-term plasticity displayed by a neocortical and a hippocampal pathway in vitro, and observed dramatic differences. Conditions known to increase transmitter release probability were more effective in hippocampus, while conditions known to decrease release probability were similarly effective in both pathways. The effects of the irreversible open-channel blocker of N-methyl-d-aspartate receptors, MK-801, implied that synapses in the neocortical pathway have a relatively high probability of transmitter release as compared with synapses in the hippocampal pathway. Differences in release probability may explain the pathway-specific variance in short- and long-term synaptic plasticity.
The functional characteristics of excitatory synapses differ significantly between pathways and brain regions. The most intensively studied synapses of the hippocampus are those formed by the Schaffer collaterals onto the dendrites of CA1 pyramidal cells, within stratum radiatum (here called, simply, the “hippocampus pathway”). In the neocortex, stimulating layer IV activates synapses on layer III pyramidal cells (here called the “neocortex pathway”). Both of these pathways produce lasting synaptic plasticity, such as long-term potentiation (LTP) and long-term depression (refs. 1–4). However, distinct differences in the expression of long-term synaptic plasticity are evident between pathways of the hippocampus and neocortex (5). Most notably, the amount of enhancement observed in the hippocampus pathway is greater and develops faster than the enhancement observed in the neocortex pathway, even when using an identical tetanizing induction protocol. The basic synaptic properties that explain these differences in long-term plasticity are unknown. One possibility is that the baseline probability of transmitter release is very different in the pathways (6). The expression of LTP may depend on an increase in the probability of transmitter release in some synapses (7), or it may contribute to LTP in other synapses together with a postsynaptic modification (8, 9). Thus, an already high probability of release in naive synapses of neocortex could limit their capacity for strong expression of LTP.
The short-term plasticity of synapses in hippocampal and neocortical pathways also varies widely. There are several forms of short-term plasticity, such as facilitation, depression, augmentation, and potentiation, that seem to involve presynaptic mechanisms (10–12). Facilitation is caused by a transient increase in the probability of transmitter release following synaptic activation, and apparently depends on a small, residual increase in presynaptic internal [Ca2+] (10, 13). Synapses that have an inherently low probability of release tend to display large facilitation, because it is more likely that the second of a stimulus pair will evoke release. In contrast, synapses with an inherently high probability of release tend to display depression, presumably because the pool of vesicles available for release becomes depleted after an initial successful release (14, 15). A different source of short-term synaptic depression can be neuromodulators (16–18) that are released by the first stimulus. These may act presynaptically to reduce the probability of release from a second stimulus. Although the hippocampal pathway shows strong facilitation, most neocortical pathways onto pyramidal cells display depression (18–19). Posttetanic potentiation, another form of short-term plasticity that is also expressed presynaptically, is largely absent in the neocortex pathway but is quite potent in the hippocampus pathway (3–5).
In this study, we compared the short-term plasticity displayed by the neocortex and hippocampus pathways under identical conditions and found them to be dramatically different. Conditions known to increase release probability were more effective in hippocampus, while conditions known to decrease release probability were similarly effective in both pathways. Using the open-channel blocker of N-methyl-d-aspartate (NMDA) receptors, MK-801, we found that the large majority of synapses in the neocortex pathway may have a high probability of release.
MATERIALS AND METHODS
The methods used in this study were similar to those described previously (4, 20). Sprague-Dawley rats (>200 g) were given an overdose of sodium pentobarbital and decapitated immediately after the disappearance of tail pinch reflexes. The brain was rapidly removed and immersed in ice-cold dissection buffer containing 124 mM NaCl, 5 mM KCl, 1.25 mM NaH2PO4, 2 mM MgSO4, 2 mM CaCl2, 26 mM NaHCO3, and 10 mM dextrose. A block containing the parietal neocortex and the underlying dorsal hippocampus was removed and sectioned coronally into 0.4-mm-thick slices using a Microslicer (DSK, Ted Pella, Redding, CA). The slices were collected and transferred to an interface slice chamber (Medical Systems, Greenvale, NY) where they were maintained in an atmosphere of humidified 95% O2/5% CO2 and superfused with the previously described buffer at 35.5°C at a rate of 1.5 ml/min. The slices were left undisturbed for at least 1 hr before recording.
Extracellular recording microelectrodes (1 MΩ) were filled with buffer solution. Stimulation was applied to layer IV in the granular somatosensory cortex, and to the Schaffer collaterals of CA1, and recordings were made from layer III and stratum radiatum of CA1, respectively. Synaptic responses were evoked with 200-μsec-long shocks of 10–60 μA delivered using a bipolar stimulating electrode. Baseline responses were obtained at 0.1 Hz using a stimulation intensity that produced approximately a one-half maximal field potential.
Synaptic responses were sampled at 5–10 kHz and stored on a computer using experimenters workbench (Data Wave Technologies, Longmont, CO). The slope of the field potentials were calculated as previously described (4) and used as a measure of the synaptic efficacy. Steady baseline measurements for at least 10 min was a requirement for any experiment to be initiated. Statistical comparisons were performed with a t test.
When drugs were used, they were either dissolved in the recording pipette solution or in the bathing solution. α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors were blocked by including 10 μM 6,7-dintroquinoxaline-2,3-dione (DNQX) in the bath solution. NMDA receptors were blocked by including 50–100 μM AP5 in the bath solution. γ-Aminobutyric acid type A (GABAA) receptors were blocked by including 5–10 mM bicuculline methiodide in the recording pipette (4, 20). GABAB receptors were blocked by including 300–500 μM 2-hydroxy-saclophen in the bath solution. MK-801 was used at 40 μM in the bath solution. During the initial 10 min of MK-801 application stimulation was halted. The drugs used were purchased from Sigma or Research Biochemicals (Natick, MA).
RESULTS
Short-Term Synaptic Plasticity Differs in the Neocortex and Hippocampus Pathways.
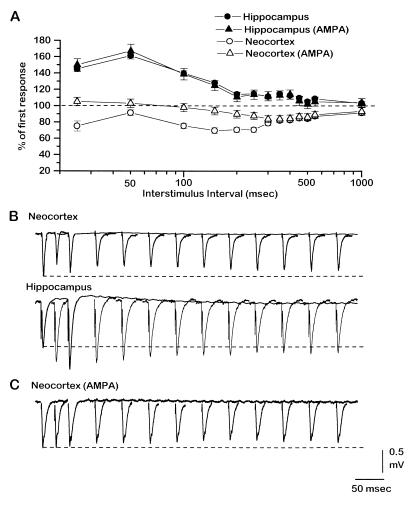
All experiments reported here were performed on coronal slices that contained both the dorsal hippocampus and the granular somatosensory neocortex. We recorded evoked field potentials to compare, under identical conditions, the short-term plasticity of two synaptic pathways within each cortical area. Unless otherwise indicated, the initial slope of the field negativity is used as a measurement (see Materials and Methods). As shown in Fig. 1 A and B (solid symbols; n = 10), paired-pulse stimulation applied to the hippocampal pathway produced significant facilitation at interstimulus intervals between 25 and 150 msec, with a peak effect at about 50 msec. In contrast, application of the same stimulus protocol to the same slices in the layer-IV-to-layer-III neocortex pathway produced depression (Fig. 1 A and B, ○; n = 10); the response to the second stimulus was depressed at all intervals between 25 and 1,000 msec. In the neocortex, paired-pulse depression was biphasic, with two peaks at about 25 and 200 msec. Blocking GABAA, GABAB, and NMDA receptors pharmacologically (AMPA in Fig. 1 A and C) had no significant effect on paired-pulse facilitation in hippocampus (Fig. 1A, ▴; n = 4), but in neocortex it eliminated the depression for a range of intervals between about 25 and 250 msec (Fig. 1 A and C, ▵; n = 7). A longer-lasting depression in neocortex, which develops at intervals greater than about 200 msec, was not abolished by receptor blockade. These results indicate that under normal conditions, and under conditions in which GABAergic and NMDA receptors are not activated, short-term plasticity differs dramatically between the tested pathways in neocortex and hippocampus.
Figure 1.
Short-term synaptic plasticity differs in the neocortex and hippocampus pathways. (A) Percentage change of the response to the second stimulus in a pair with respect to the first as a function of interstimulus interval, in the hippocampus (solid symbols) and neocortex (open symbols) pathways during control conditions (circles) and after blockade of GABAA, GABAB, and NMDA receptors (triangles). Points are means of 10 experiments ± SEM. (B) Representative examples of evoked field potentials from the neocortex (Upper) and hippocampus (Lower) pathways under control conditions. Overlayed are the response to the first stimulus and the responses to the second stimulus at different interstimulus intervals (25, 50, 100, 150, 200, 250, 300, 350, 400, 450, 500, and 550 msec). Dashed lines show amplitude of first response for reference. (C) Representative examples of evoked field potentials from the neocortex during blockade of GABAA, GABAB, and NMDA receptors, stimulated as in B. After drugs were applied to block these receptors the stimulation was lowered to match the amplitude of the first response in the pre-drug condition. This change in stimulation strength did not affect the paired-pulse ratio.
[Ca2+]o Differentially Affects Short-Term Plasticity in the Two Pathways.
Modifications of [Ca2+]o influence the probability of transmitter release and short-term synaptic plasticity (13). We used [Ca2+]o changes to alter the dynamics of evoked responses in the neocortex and hippocampus pathways. Fig. 2A shows an example of the effect of increasing [Ca2+]o from 2 to 5 mM, while recording simultaneously from the hippocampus (▴) and neocortex (▵) in the same slice. Effects in the two pathways differed: neocortex showed only a small enhancement of its evoked response, while the hippocampus was much more strongly and significantly enhanced (neocortex vs. hippocampus; P < 0.0001). Fig. 2B shows the average of five experiments for the neocortex (open bars) and hippocampus (solid bars) under drug-free conditions (Normal) and during blockade of GABAA, GABAB, and NMDA receptors (AMPA). The differential effect on pathways observed after increasing [Ca2+]o did not differ significantly between the two conditions (i.e., AMPA vs. Normal; Fig. 2B).
Figure 2.
[Ca2+]o differentially affects short-term plasticity in the neocortex and hippocampus pathways. (A) Representative examples of response change (slope) as [Ca2+]o is increased (triangles) or decreased (squares) in the neocortex (open symbols) and in the hippocampus (solid symbols) pathways. The arrow indicates the moment of [Ca2+]o change from a control condition of 2 mM to 5 mM (triangles) or to 1 mM (squares). Plotted is the percentage change of the evoked field potentials relative to the average response in the control condition. (B) Histogram summarizing the results in A from five experiments per group, during control conditions (Normal) and during blockade of GABAA, GABAB, and NMDA receptors (AMPA) ± SEM. The open bars are data from neocortex; solid bars are from hippocampus. (C) Percentage change of the response to the second stimulus in a pair with respect to the first as a function of interstimulus interval and [Ca2+]o, in the hippocampus (solid symbols) and neocortex (open symbols) pathways during control conditions (Left, Normal) and after blockade of GABAA, GABAB, and NMDA receptors (Right, AMPA). Results are averages of five experiments per group (SEMs < 5%; not shown).
Fig. 2C shows that the changes in synaptic efficacy induced by increasing [Ca2+]o from 2 mM (circles) to 5 mM (triangles) were paralleled by modifications in short-term plasticity. The average responses from five experiments were plotted for the neocortex (open symbols) and hippocampus (solid symbols), under drug-free conditions (Left; Normal) and for the neocortex during blockade of GABAA, GABAB, and NMDA receptors (Right; AMPA). After increasing [Ca2+]o, the neocortex (Fig. 2C, Normal; ▵) showed stronger depression at 25- to 50-msec intervals, while the hippocampus showed a suppressed facilitation at those same intervals (Fig. 2C Normal; ▴). When GABAA, GABAB, and NMDA receptors were blocked in neocortex at 2 mM [Ca2+]o, depression decreased at the initial intervals (as described above). When [Ca2+]o was increased with receptors blocked, depression still developed at 25- to 50-msec intervals (Fig. 2C, AMPA; ▵). During blockade of GABAA, GABAB, and NMDA receptors in hippocampus the effects of increasing [Ca2+]o on short-term plasticity did not differ significantly from the drug-free condition (not shown).
Facilitation Is Revealed in Neocortex when Probability of Release Is Reduced.
In contrast with the differential effects observed after increasing [Ca2+]o, decreases in [Ca2+]o from 2 to 1 mM produced a similar result in both pathways: the size of the evoked responses decreased (Fig. 2A, squares). The decrease in response size did not differ significantly between the two pathways and was not affected when GABAA, GABAB, and NMDA receptors were blocked (Fig. 2B). The change in the size of the evoked response produced by the [Ca2+]o decrease was accompanied by an enhancement in paired-pulse facilitation at 25- to 50-msec intervals in the hippocampus pathway (Fig. 2C, Normal; ▪), and by an appearance of facilitation in the neocortex pathway (Fig. 2C, Normal; □). Similar results also occurred during blockade of GABAA, GABAB, and NMDA receptors in neocortex (Fig. 2C, AMPA; □), and in hippocampus (not shown).
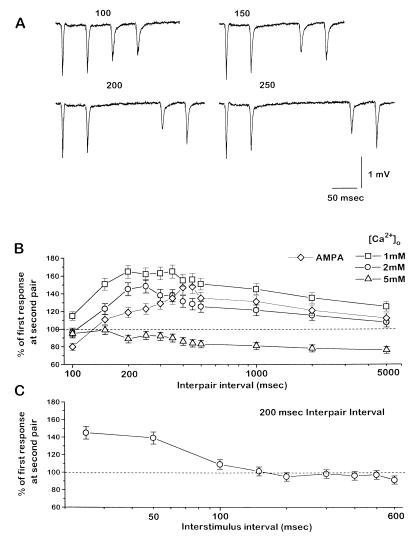
The fact that the evoked response did not increase in the neocortex pathway as much as in the hippocampus pathway during high [Ca2+]o, but decreased similarly in both pathways during low [Ca2+]o, suggests that the probability of transmitter release from the neocortical synapses is generally higher than that in the hippocampal synapses. Moreover, the existence of a long-lasting depression in neocortex that is unaffected by changes in [Ca2+]o (>200-msec intervals; Fig. 2C) suggests that some modulator(s) might be decreasing release probability after the first stimulus, and thus contributing to paired-pulse depression there. The lack of effect of [Ca2+]o on this long-lasting depression implies that if a decrease in release probability is involved, it must occur at a site downstream from presynaptic calcium entry (21). Consistent with the long-lasting depression involving a decrease in release probability, paired pulses delivered during this depression express facilitation. Fig. 3A shows the effect of varying the interval between two sequential pairs of stimuli, each delivered with an interstimulus interval of 50 msec. As the interpair interval increased, two results were apparent. The absolute size of the responses to the second pair became smaller (as a result of the long-lasting depression shown in Fig. 1), but paired-pulse facilitation developed in that pair. Facilitation occurred for a long period of interpair intervals, beginning at about 200 msec and lasting up to several seconds (Fig. 3B). Varying the interstimulus interval of the second pair of pulses revealed that facilitation peaked at 25–50 msec (Fig. 3C), similar to that in hippocampus and in neocortex in low [Ca2+]o.
Figure 3.
Facilitation is revealed in neocortex with pairs of paired stimuli. (A) Pairs of paired stimuli were applied. Representative responses from varying the interval between two pairs of stimuli (interpair interval) in the neocortex. Each pair has an interstimulus interval of 50 msec, and the interval between pairs is 100, 150, 200, and 250 msec. Note the facilitation developing in the second pair as interpair interval increases. (B) Duration of conditioning-induced facilitation. Percentage change of the response to the second stimulus in the second pair with respect to the first response in the second pair as a function of interpair interval during 1 mM (squares), 2 mM (circles), and 5 mM (triangles) [Ca2+]o, and during block of GABAA, GABAB, and NMDA receptors (diamonds). The interval between stimuli within each pair was 50 msec. Shown are the mean ± SEM of 4–5 experiments per group. (C) Interval dependence of conditioning-induced facilitation. Percentage of change of the response to the second stimulus in the second pair with respect to the first response in the second pair as a function of the interstimulus interval in the second pair, during a fixed 200-msec interpair interval and control conditions.
Increasing [Ca2+]o to 5 mM readily abolished this paired-pair facilitation (Fig. 3B, triangles), while decreasing [Ca2+]o to 1 mM enhanced facilitation (Fig. 3B, squares). The facilitation revealed by this paired-pair protocol was also observed after blockade of GABAA, GABAB, and NMDA receptors (Fig. 3B, diamonds). Similar paired-pair stimuli applied to the hippocampus pathway simply produced paired-pulse facilitation to a similar degree in pairs delivered at intervals greater than about 200 msec (i.e., the first response to the second stimulus pair needed this interpair interval to recover from the facilitation produced by the first pair; data not shown).
Most Synapses in the Neocortex Pathway Have a Relatively High Probability of Release.
Several results in this study suggest that synapses in the neocortex pathway have, on average, a higher probability of release than those in the hippocampus pathway. First, under control conditions paired-pulse depression is evoked in the neocortex, while facilitation is evoked in the hippocampus pathway. Second, increasing [Ca2+]o (a manipulation known to increase release probability) was much less effective at increasing transmitter release in the neocortex than it was in the hippocampus. Finally, facilitation was unmasked in neocortex by conditions likely to decrease release probability. Previous studies have shown that most of the synapses in the hippocampus pathway of young rats have a very low probability of release (6, 16, 17), but no data are available on neocortex.
To test further the hypothesis that most synapses in the neocortex pathway have a relatively high release probability, we used the irreversible open-channel blocking action of the NMDA receptor antagonist MK-801. Release of transmitter from a synapse in the presence of MK-801 results in essentially permanent blockade of the opened proportion of its NMDA receptors; repeated activation of a group of synapses results in a progressive decline of the amplitude of the population NMDA receptor-mediated synaptic potential as NMDA channels are removed from the available pool. The rate of decline of the potential thus depends on the probability of transmitter release (6, 16, 17), other things being equal (see below).
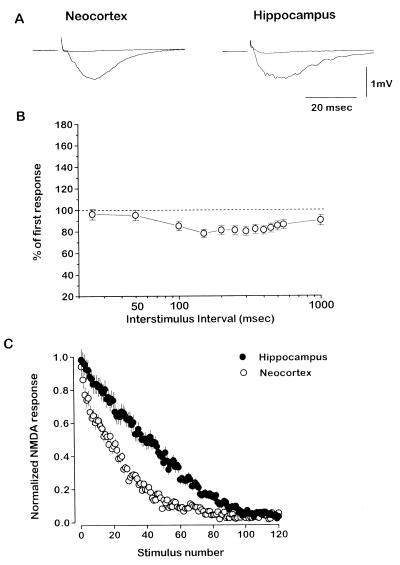
We measured, simultaneously in the two pathways, an NMDA receptor-mediated field potential response isolated by blocking AMPA receptors and GABAA receptors and lowering [Mg2+]o to 1 mM (Fig. 4A). Fig. 4B shows that isolated NMDA-mediated responses in neocortex had paired-pulse profiles similar to those of AMPA-mediated responses to pairs of pulses (cf. Fig. 1); with paired-pair stimulus protocols, facilitation was also unmasked at the same intervals (not shown). After MK-801 (40 μM) was washed into the bath, repetitive stimulation was applied to both pathways at 0.1 Hz and the rate of decay of the NMDA responses was measured. The decay rate was significantly faster in the neocortex pathway (130 ± 9 sec to half-amplitude) than in the hippocampus pathway (297 ± 12 sec stimuli at half-decay) (Fig. 4B; n = 10 slices, P < 0.0001).
Figure 4.
Block of NMDA receptor-mediated responses by MK-801 is faster in neocortex than in hippocampus. (A) Representative examples of NMDA receptor-mediated field potentials in the neocortex (Left) and in the hippocampus (Right) pathways before and after activity-dependent block with MK-801. Slices were bathed in CNQX and 1 mM [Mg2+]o, and recording electrodes contained bicuculline. (B) Percentage change of NMDA receptor-mediated responses to the second stimulus in a pair with respect to the first as a function of interstimulus interval, in the neocortex (n = 3; mean ± SEM). (C) Normalized NMDA receptor-mediated responses (amplitude) in the neocortex (•) and in the hippocampus (○) pathways as single stimuli were delivered at 0.1 Hz in the presence of MK-801 (n = 10 per group; mean ± SEM).
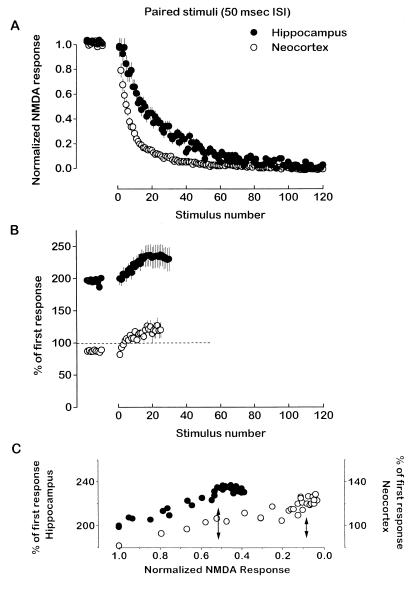
Two straightforward possibilities can explain this result. First, the proportion of synapses with a relatively high probability of release may be larger in the neocortex pathway than in the hippocampus pathway. Alternatively, neocortex would decline faster if it had a higher fraction of NMDA channels blocked by MK-801 when a synapse released transmitter (6, 16). The fractional block of NMDA channels has not been measured in neocortical synapses. To help distinguish between the two possibilities, we measured the change in paired-pulse responses (50-msec interstimulus interval, applied at 0.1 Hz) in both pathways during application of MK-801. Once again the rate of decay was significantly faster in the neocortex (55 ± 6 sec at half-decay) than in the hippocampus (115 ± 8 sec at half-decay) (Fig. 5A; n = 7 slices), and the decay rates with stimulus pairs were about twice as fast as when single stimuli were applied in the presence of MK-801 (cf. Fig. 4C). As the NMDA responses declined in the presence of MK-801, paired-pulse ratios shifted to more facilitated levels in both pathways, although the hippocampal synapses started from a high baseline level of facilitation while the neocortical synapses started with a slight depression (Fig. 5B). Facilitation in both pathways peaked by about stimulus pair 18, and then stabilized at about 240% for the hippocampus and 120% for neocortex. We reasoned that, in each synapse pool, those synapses with highest probability of release would be the first ones blocked by the drug; sequential measurements of the paired-pulse relationship as synapses were blocked would ultimately reveal those synapses with the strongest facilitation, and thus the lowest probability of release. This measure is independent of the fractional block per active synapse.
Figure 5.
Most synapses in the neocortex pathway have a relatively high probability of release. (A) Normalized NMDA receptor-mediated responses (amplitude) in the neocortex (•) and in the hippocampus (○) pathways as paired stimuli [50 msec interstimulus interval (ISI)] were delivered at 0.1 Hz before and in the presence of MK-801 (n = 7 per group; mean ± SEM). (B) MK-801 increases the facilitation ratio in both pathways. Percentage change of the NMDA receptor-mediated response to the second stimulus in a pair with respect to the first as a function of the pair number (results are from experiments in A), before and in the presence of MK-801 (n = 7 per group; mean ± SEM). (C) Percentage change of the NMDA receptor-mediated response to the second stimulus in a pair with respect to the first as a function of the normalized amplitude of the NMDA receptor-mediated response in hippocampus (left axis, •) and in neocortex (right axis, ○). Arrows indicate the amplitude of NMDA receptor-mediated response when facilitation reached its maximum plateau level for hippocampus and neocortex.
If the percentage block of the NMDA receptor-mediated response is considered relative to the peak in paired-pulse facilitation (Fig. 5C), synapses carrying about 90% of the current in the neocortex pathway and synapses carrying about 50% of the current in the hippocampus pathway behave as if they have the highest probability of release in their pool. This follows because about 90% of the NMDA response is blocked in neocortex when its maximum paired-pulse facilitation is reached, while about 50% of the NMDA response is blocked in hippocampus when it reaches its maximum facilitation. If the primary difference between the two pathways had been the fractional blockade of channels per releasing synapse, and not the probability of transmitter release, then it is unlikely MK-801 would reveal further facilitation in both pathways. The large difference (twofold) in the maximal facilitation of the two pathways implies further that the synapses carrying half of the current in hippocampus have a much lower release probability than the synapses carrying roughly 10% of the current in neocortex with the lowest release probability.
DISCUSSION
The results of this study suggest that the large majority of synapses in the excitatory neocortex pathway (layer IV-to-layer III) have a relatively high probability of release, while synapses in the hippocampus pathway (Schaffer collaterals of CA1) have a substantially lower probability of release. This difference in basic release properties may explain the distinct characteristics of short-term and long-term forms of plasticity in the two pathways. Thus, a high release probability in naive synapses of neocortex would hinder paired-pulse facilitation, posttetanic potentiation, and a presynaptic contribution to LTP. Indeed, these are all characteristic features of plasticity in the neocortex pathway investigated here.
Previous Work on the Hippocampus and Neocortex Pathways.
The release properties and plasticity of hippocampal synapses have been well studied, and our results are in close agreement with previous work. Hippocampal synapses generate robust paired-pulse facilitation, and increasing [Ca2+]o abolishes facilitation and increases the size of the evoked response due to an increase in the probability of release (22). Moreover, our data from the application of MK-801 to hippocampus produced results similar to those reported by others (6, 16, 17), considering the wide differences in methods. Data from hippocampal slices from animals younger than those used here (6, 17) suggested two populations, or a range, of synapses based on release probability: low and high probability groups, with the low probability portion accounting for half or more of the total.
In contrast with the hippocampus, MK-801 has never been used to test the probability of release in the neocortex. The short-term plasticity of excitatory synapses onto most pyramidal cells in the neocortex tend to show paired-pulse depression (18, 19, 23–25), but there are exceptions (26, 27). In cat visual cortex, different types of excitatory synapses onto layer IV spiny stellate cells show distinct strengths and short-term plasticity (28). Studies of synaptically coupled pairs of neocortical neurons show that trial-to-trial transmission failure rates are also variable among different pathways of the neocortex (19, 28).
Conclusions and Implications.
We found that depression was evoked in the neocortex pathway in response to paired stimuli, and that increasing [Ca2+]o had only a slight effect on the evoked synaptic response. A simple interpretation of these results is that the majority of synapses in this pathway have a high release probability in normal [Ca2+]o, and therefore cannot be induced to increase their release much further. A small proportion of the synapses in the neocortex pathway may have a low release probability; increasing [Ca2+]o enhanced paired-pulse depression at those intervals (25–50 msec) where facilitation was expressed under other conditions (e.g., Fig. 3C), suggesting that this facilitation was masked by the larger proportion of synapses displaying depression. In contrast, lowering [Ca2+]o, a manipulation known to decrease release probability, produced a decrease of the evoked response and unmasked paired-pulse facilitation in the neocortical pathway. Taken together, these results suggest that the probability of release can be significantly reduced in these neocortical synapses, but cannot be much further enhanced because of an already high probability of release.
Paired-pulse facilitation could be unmasked in the neocortex by delivering a priming stimulus about 200 msec before the test pair of stimuli (Fig. 3). This revealed facilitation has the properties of presynaptically mediated facilitation, because it was selectively abolished by increasing [Ca2+]o, and enhanced by decreasing [Ca2+]o. Thus, priming stimuli apparently cause a decrease in release probability lasting for several seconds. The mechanism is unknown, but seems likely to involve the release of one or more neuromodulators that act presynaptically (16, 21, 22, 29, 30). The identity of this modulator is also unknown, but there are myriad candidates (e.g., refs. 22, 29–31). Other mechanisms not involving a modulator are certainly possible.
MK-801 was used as an alternative way to probe the release probability of the synapses tested. When stimulated at a low frequency, MK-801 blocked NMDA receptor-mediated transmission much faster in the neocortex than in the hippocampus pathway. This result can also be explained if most of the neocortical synapses have a relatively high probability of release. Indeed our rough estimates suggest that more than 90% of the current in the neocortex pathway is carried by synapses that have a substantially higher release probability than the synapses carrying 50% of the current in the hippocampal pathway, with the highest release probability there. An alternative interpretation of the MK-801 result is that NMDA channels in hippocampus and neocortex have very different probabilities of opening when a synapse has released transmitter. This seems unlikely to explain our results, because the facilitation ratio of the two pathways shifted similarly toward greater facilitation as MK-801 blockade progressed, and the kinetics of synaptically generated NMDA currents in relatively mature neocortex (32, 33) and hippocampus (6, 16) are roughly similar.
A distinct difference in transmitter release probability could underlie several of the characteristic differences in short- and long-term synaptic plasticity between hippocampus and neocortex (5). Here we found that facilitation is only expressed in the hippocampus and not in the layer-IV-to-layer-III pathway in neocortex under normal conditions. Because facilitation mainly involves an increase in release probability in synapses with an inherently low release probability, facilitation could not be strongly expressed in the neocortex pathway. Previous work on LTP in neocortex typically showed that its magnitude of expression is small (3–5). This could also be explained if the magnitude of LTP is limited by the baseline properties of presynaptic release. Currently, there is disagreement about whether NMDA receptor-dependent LTP has a presynaptic or postsynaptic locus of expression (34, 35), and it is possible that there are contributions at both sites. A synaptic population with a generally high release probability would necessarily limit LTP expression in neocortex mostly to a postsynaptic site of expression, and/or to the small population of synapses with a low baseline release probability. Finally, previous studies have also pointed out that posttetanic potentiation is small or absent in neocortex (3–5), and this could also be attributed to a high release probability in this pathway.
Our results reinforce observations that basic synaptic physiology can vary widely in different types of synapses in the cerebral cortex (28, 36, 37). The structural and molecular bases for these differences are unknown. The size of presynaptic boutons may correlate with release probability (38); ultrastructural studies of neocortical synapses show that presynaptic terminals have a wide range of diameters, and terminal size may be pathway-specific (39). Thus, the release characteristics and plasticity of synapses in neocortex may be adapted to the specific requirements of each pathway.
Acknowledgments
This study was supported by fellowships to M.A.C. from the Ministry of Science and Education of Spain, the National Institute of Mental Health (MH19118), and the Epilepsy Foundation of America, and by a grant to B.W.C. from the National Institutes of Health (NS25983).
ABBREVIATIONS
- NMDA
N-methyl-d-aspartate
- LTP
long-term potentiation
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- GABA
γ-aminobutyric acid
References
- 1.Bliss T V P, Collingridge G L. Nature (London) 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 2.Bear M F, Malenka R C. Curr Opin Neurobiol. 1994;4:389–399. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 3.Kirkwood A, Dudek S M, Gold J T, Aizenman C D, Bear M F. Science. 1993;260:1518–1521. doi: 10.1126/science.8502997. [DOI] [PubMed] [Google Scholar]
- 4.Castro-Alamancos M A, Donoghue J P, Connors B W. J Neurosci. 1995;15:5324–5333. doi: 10.1523/JNEUROSCI.15-07-05324.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malenka R C. In: The Cortical Neuron. Gutnick M J, Mody I, editors. New York: Oxford Univ. Press; 1995. pp. 98–110. [Google Scholar]
- 6.Hessler N A, Shirke A M, Malinow R. Nature (London) 1993;366:569–572. doi: 10.1038/366569a0. [DOI] [PubMed] [Google Scholar]
- 7.Weisskopf M G, Nicoll R A. Nature (London) 1995;376:256–259. doi: 10.1038/376256a0. [DOI] [PubMed] [Google Scholar]
- 8.Kullman D M, Erdemli G, Asztely F. Neuron. 1996;17:461–474. doi: 10.1016/s0896-6273(00)80178-6. [DOI] [PubMed] [Google Scholar]
- 9.Oliet S H R, Malenka R C, Nicoll R A. Science. 1996;271:1294–1297. doi: 10.1126/science.271.5253.1294. [DOI] [PubMed] [Google Scholar]
- 10.Zucker R S. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]
- 11.Kamiya H, Zucker R S. Nature (London) 1994;371:603–606. doi: 10.1038/371603a0. [DOI] [PubMed] [Google Scholar]
- 12.Zengel J E, Magleby K L, Horn J P, McAfee D A, Yarowsky P J. J Gen Physiol. 1980;76:213–231. doi: 10.1085/jgp.76.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katz B, Miledi R. J Physiol (London) 1968;195:481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevens C F, Wang Y. Neuron. 1995;14:795–802. doi: 10.1016/0896-6273(95)90223-6. [DOI] [PubMed] [Google Scholar]
- 15.Debanne D, Guerineau N C, Gahwiler B H, Thompson S M. J Physiol (London) 1996;491:163–176. doi: 10.1113/jphysiol.1996.sp021204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenmund C, Clements J D, Westbrook G L. Science. 1993;262:754–757. doi: 10.1126/science.7901909. [DOI] [PubMed] [Google Scholar]
- 17.Manabe T, Nicoll R A. Science. 1994;265:1888–1892. doi: 10.1126/science.7916483. [DOI] [PubMed] [Google Scholar]
- 18.Thomson A M, West D C, Deuchars J. In: Excitatory Amino Acids and the Cerebral Cortex. Conti F, Hicks T P, editors. Cambridge, MA: MIT Press; 1996. pp. 99–108. [Google Scholar]
- 19.Volgushev M, Voronin L L, Chistiakova M, Artola A, Singer W. Eur J Neurosci. 1995;7:1751–1760. doi: 10.1111/j.1460-9568.1995.tb00695.x. [DOI] [PubMed] [Google Scholar]
- 20.Castro-Alamancos M A, Connors B W. Proc Natl Acad Sci USA. 1996;93:1335–1339. doi: 10.1073/pnas.93.3.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson S M, Capogna M, Scanziani M. Trends Neurosci. 1993;16:222–227. doi: 10.1016/0166-2236(93)90160-n. [DOI] [PubMed] [Google Scholar]
- 22.Manabe T, Wyllie D J A, Perkel D J, Nicoll R A. J Neurophysiol. 1993;70:1451–1459. doi: 10.1152/jn.1993.70.4.1451. [DOI] [PubMed] [Google Scholar]
- 23.Thomson A M, Deuchars J, West D C. J Neurophysiol. 1993;70:2354–2369. doi: 10.1152/jn.1993.70.6.2354. [DOI] [PubMed] [Google Scholar]
- 24.Deuchars J, West D C, Thomson A M. J Physiol (Lond) 1994;478:423–435. doi: 10.1113/jphysiol.1994.sp020262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Markram H, Tsodyks M. Nature (London) 1996;382:807–810. doi: 10.1038/382807a0. [DOI] [PubMed] [Google Scholar]
- 26.Thomson A M, West D C, Deuchars J. Neuroscience. 1995;69:727–738. doi: 10.1016/0306-4522(95)00287-s. [DOI] [PubMed] [Google Scholar]
- 27.Deuchars J, Thomson A M. Neuroscience. 1995;69:739–755. doi: 10.1016/0306-4522(95)00288-t. [DOI] [PubMed] [Google Scholar]
- 28.Stratford K J, Tarczy-Hornoch K, Martin K A C, Bannister N J, Jack J J B. Nature (London) 1996;382:258–261. doi: 10.1038/382258a0. [DOI] [PubMed] [Google Scholar]
- 29.Manzoni O J, Manabe T, Nicoll R A. Science. 1994;265:2098–2101. doi: 10.1126/science.7916485. [DOI] [PubMed] [Google Scholar]
- 30.Kang Y. J Neurosci. 1995;15:8268–8280. doi: 10.1523/JNEUROSCI.15-12-08268.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mott D D, Lewis D V. Int Rev Neurobiol. 1994;36:97–223. doi: 10.1016/s0074-7742(08)60304-9. [DOI] [PubMed] [Google Scholar]
- 32.Carmignoto G, Vicini S. Science. 1992;258:1007–1011. doi: 10.1126/science.1279803. [DOI] [PubMed] [Google Scholar]
- 33.Crair M C, Malenka R C. Nature (London) 1995;375:325–328. doi: 10.1038/375325a0. [DOI] [PubMed] [Google Scholar]
- 34.Malenka R C, Nicoll R A. Trends Neurosci. 1993;16:521–527. doi: 10.1016/0166-2236(93)90197-t. [DOI] [PubMed] [Google Scholar]
- 35.Kullman D M, Siegelbaum S A. Neuron. 1996;15:997–1002. doi: 10.1016/0896-6273(95)90089-6. [DOI] [PubMed] [Google Scholar]
- 36.Nicoll R A, Malenka R C. Nature (London) 1995;377:115–118. doi: 10.1038/377115a0. [DOI] [PubMed] [Google Scholar]
- 37.Salin P A, Scanziani M, Malenka R C, Nicoll R A. Proc Natl Acad Sci USA. 1996;93:13304–13309. doi: 10.1073/pnas.93.23.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lisman J E, Harris K M. Trends Neurosci. 1993;16:141–147. doi: 10.1016/0166-2236(93)90122-3. [DOI] [PubMed] [Google Scholar]
- 39.Kharazia V N, Weinberg R J. J Neurosci. 1994;14:6021–6032. doi: 10.1523/JNEUROSCI.14-10-06021.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]