Abstract
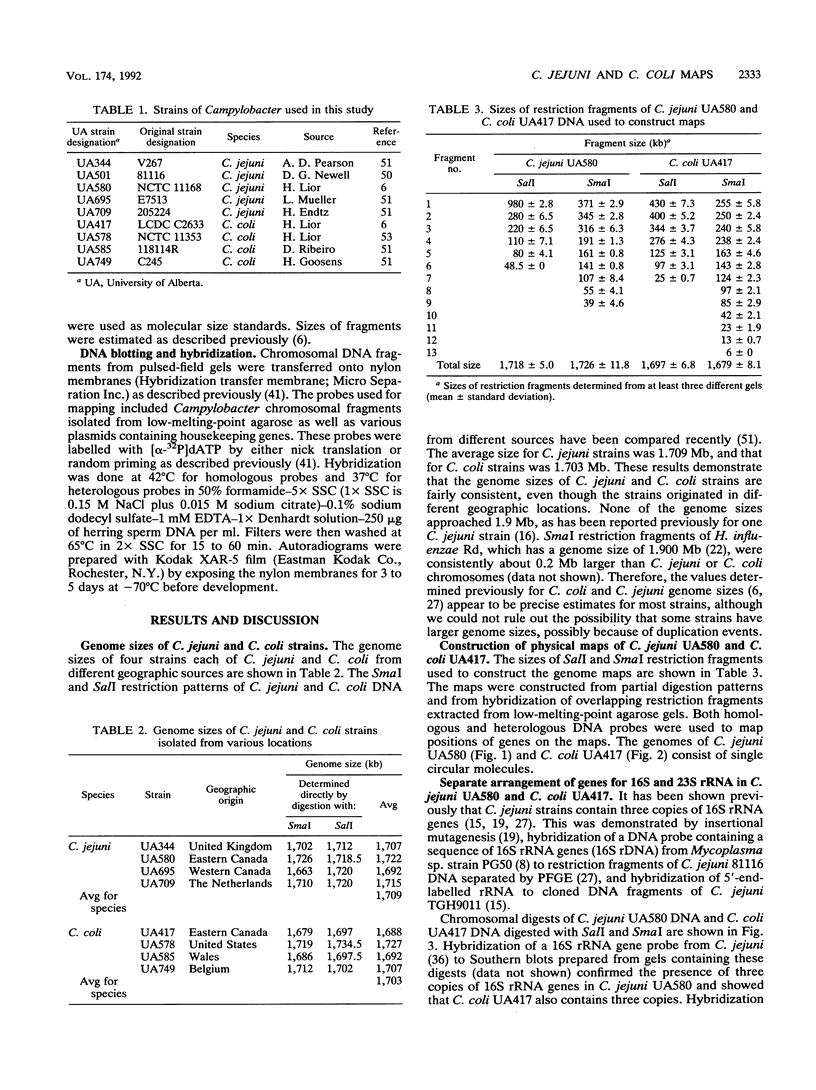
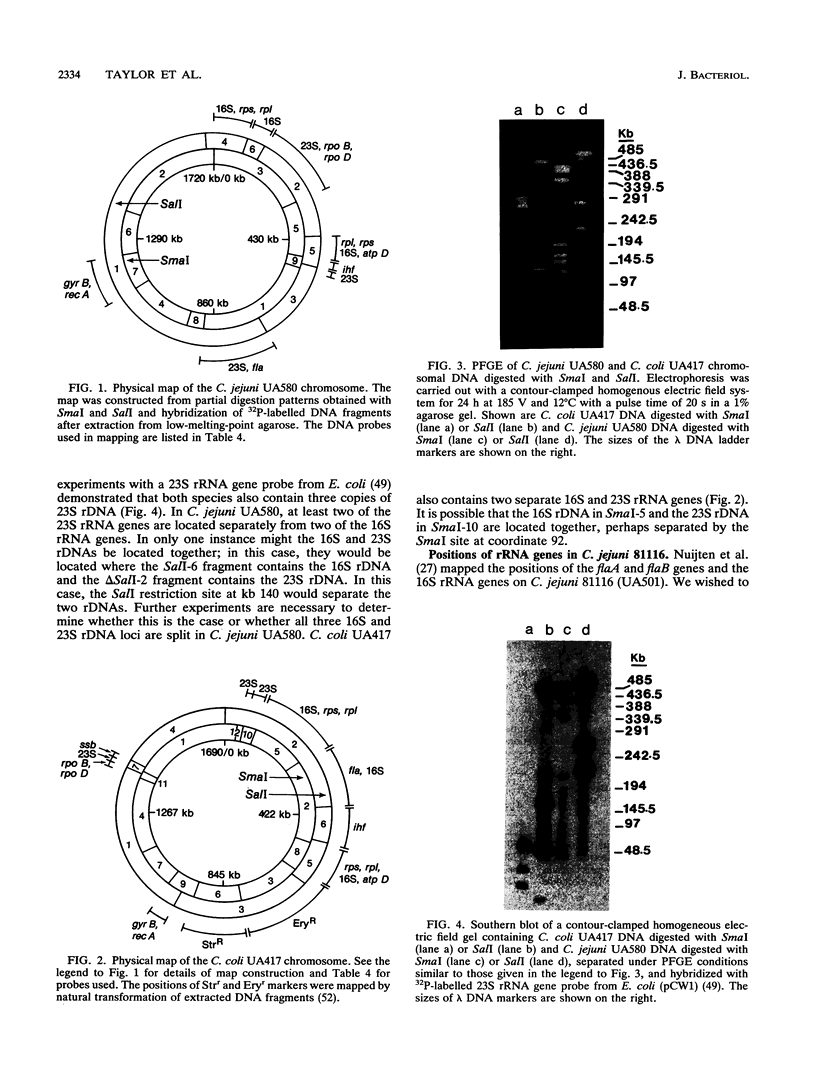
Little information concerning the genome of either Campylobacter jejuni or Campylobacter coli is available. Therefore, we constructed genomic maps of C. jejuni UA580 and C. coli UA417 by using pulsed-field gel electrophoresis. The genome sizes of C. jejuni and C. coli strains are approximately 1.7 Mb, as determined by SalI and SmaI digestion (N. Chang and D. E. Taylor, J. Bacteriol. 172:5211-5217, 1990). The genomes of both species are represented by single circular DNA molecules, and maps were constructed by partial restriction digestion and hybridization of DNA fragments extracted from low-melting-point agarose gels. Homologous DNA probes, encoding the flaAB and 16S rRNA genes, as well as heterologous DNA probes from Escherichia coli, Bacillus subtilis, and Haemophilus influenzae, were used to identify the locations of particular genes. C. jejuni and C. coli contain three copies of the 16S and 23S rRNA genes. However, they are not located together within an operon but show a distinct split in at least two of their three copies. The positions of various housekeeping genes in both C. jejuni UA580 and C. coli UA417 have been determined, and there appears to be some conservation of gene arrangement between the two species.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev. 1990 Jun;54(2):130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcak G. J., Tomb J. F., Laufer C. S., Smith H. O. Two Haemophilus influenzae Rd genes that complement the recA-like mutation rec-1. J Bacteriol. 1989 May;171(5):2451–2457. doi: 10.1128/jb.171.5.2451-2457.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler P. D., Moxon E. R. A physical map of the genome of Haemophilus influenzae type b. J Gen Microbiol. 1990 Dec;136(12):2333–2342. doi: 10.1099/00221287-136-12-2333. [DOI] [PubMed] [Google Scholar]
- Canard B., Cole S. T. Genome organization of the anaerobic pathogen Clostridium perfringens. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6676–6680. doi: 10.1073/pnas.86.17.6676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang N., Taylor D. E. Use of pulsed-field agarose gel electrophoresis to size genomes of Campylobacter species and to construct a SalI map of Campylobacter jejuni UA580. J Bacteriol. 1990 Sep;172(9):5211–5217. doi: 10.1128/jb.172.9.5211-5217.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Finch L. R. Novel arrangement of rRNA genes in Mycoplasma gallisepticum: separation of the 16S gene of one set from the 23S and 5S genes. J Bacteriol. 1989 May;171(5):2876–2878. doi: 10.1128/jb.171.5.2876-2878.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frydenberg J., Christiansen C. The sequence of 16S rRNA from Mycoplasma strain PG50. DNA. 1985 Apr;4(2):127–137. doi: 10.1089/dna.1985.4.127. [DOI] [PubMed] [Google Scholar]
- Fukunaga M., Mifuchi I. Unique organization of Leptospira interrogans rRNA genes. J Bacteriol. 1989 Nov;171(11):5763–5767. doi: 10.1128/jb.171.11.5763-5767.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., Logan S. M., Thornton S., Trust T. J. Genomic organization and expression of Campylobacter flagellin genes. J Bacteriol. 1990 Apr;172(4):1853–1860. doi: 10.1128/jb.172.4.1853-1860.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann R. K., Erdmann V. A. Thermus thermophilus 16S rRNA is transcribed from an isolated transcription unit. J Bacteriol. 1989 Jun;171(6):2933–2941. doi: 10.1128/jb.171.6.2933-2941.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann R. K., Ulbrich N., Erdmann V. A. An unusual rRNA operon constellation: in Thermus thermophilus HB8 the 23S/5S rRNA operon is a separate entity from the 16S rRNA operon. Biochimie. 1987 Oct;69(10):1097–1104. doi: 10.1016/0300-9084(87)90009-5. [DOI] [PubMed] [Google Scholar]
- Kauc L., Goodgal S. H. The size and a physical map of the chromosome of Haemophilus parainfluenzae. Gene. 1989 Nov 30;83(2):377–380. doi: 10.1016/0378-1119(89)90125-x. [DOI] [PubMed] [Google Scholar]
- Krawiec S., Riley M. Organization of the bacterial chromosome. Microbiol Rev. 1990 Dec;54(4):502–539. doi: 10.1128/mr.54.4.502-539.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labigne-Roussel A., Courcoux P., Tompkins L. Gene disruption and replacement as a feasible approach for mutagenesis of Campylobacter jejuni. J Bacteriol. 1988 Apr;170(4):1704–1708. doi: 10.1128/jb.170.4.1704-1708.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau P. P., DeBrunner-Vossbrinck B., Dunn B., Miotto K., MacDonnell M. T., Rollins D. M., Pillidge C. J., Hespell R. B., Colwell R. R., Sogin M. L. Phylogenetic diversity and position of the genus Campylobacter. Syst Appl Microbiol. 1987;9:231–238. doi: 10.1016/s0723-2020(87)80027-9. [DOI] [PubMed] [Google Scholar]
- Lee J. J., Smith H. O., Redfield R. J. Organization of the Haemophilus influenzae Rd genome. J Bacteriol. 1989 Jun;171(6):3016–3024. doi: 10.1128/jb.171.6.3016-3024.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. J., Smith H. O. Sizing of the Haemophilus influenzae Rd genome by pulsed-field agarose gel electrophoresis. J Bacteriol. 1988 Sep;170(9):4402–4405. doi: 10.1128/jb.170.9.4402-4405.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesack W., Stackebrandt E. Evidence for unlinked rrn operons in the Planctomycete Pirellula marina. J Bacteriol. 1989 Sep;171(9):5025–5030. doi: 10.1128/jb.171.9.5025-5030.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohman T. M., Green J. M., Beyer R. S. Large-scale overproduction and rapid purification of the Escherichia coli ssb gene product. Expression of the ssb gene under lambda PL control. Biochemistry. 1986 Jan 14;25(1):21–25. doi: 10.1021/bi00349a004. [DOI] [PubMed] [Google Scholar]
- Loughney K., Lund E., Dahlberg J. E. tRNA genes are found between 16S and 23S rRNA genes in Bacillus subtilis. Nucleic Acids Res. 1982 Mar 11;10(5):1607–1624. doi: 10.1093/nar/10.5.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash H. A., Robertson C. A., Flamm E., Weisberg R. A., Miller H. I. Overproduction of Escherichia coli integration host factor, a protein with nonidentical subunits. J Bacteriol. 1987 Sep;169(9):4124–4127. doi: 10.1128/jb.169.9.4124-4127.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuijten P. J., Bartels C., Bleumink-Pluym N. M., Gaastra W., van der Zeijst B. A. Size and physical map of the Campylobacter jejuni chromosome. Nucleic Acids Res. 1990 Nov 11;18(21):6211–6214. doi: 10.1093/nar/18.21.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuijten P. J., Bleumink-Pluym N. M., Gaastra W., van der Zeijst B. A. Flagellin expression in Campylobacter jejuni is regulated at the transcriptional level. Infect Immun. 1989 Apr;57(4):1084–1088. doi: 10.1128/iai.57.4.1084-1088.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuijten P. J., van Asten F. J., Gaastra W., van der Zeijst B. A. Structural and functional analysis of two Campylobacter jejuni flagellin genes. J Biol Chem. 1990 Oct 15;265(29):17798–17804. [PubMed] [Google Scholar]
- Okada N., Sasakawa C., Tobe T., Talukder K. A., Komatsu K., Yoshikawa M. Construction of a physical map of the chromosome of Shigella flexneri 2a and the direct assignment of nine virulence-associated loci identified by Tn5 insertions. Mol Microbiol. 1991 Sep;5(9):2171–2180. doi: 10.1111/j.1365-2958.1991.tb02147.x. [DOI] [PubMed] [Google Scholar]
- Post L. E., Nomura M. DNA sequences from the str operon of Escherichia coli. J Biol Chem. 1980 May 25;255(10):4660–4666. [PubMed] [Google Scholar]
- Post L. E., Strycharz G. D., Nomura M., Lewis H., Dennis P. P. Nucleotide sequence of the ribosomal protein gene cluster adjacent to the gene for RNA polymerase subunit beta in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1697–1701. doi: 10.1073/pnas.76.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C. W., Doi R. H. Genetic mapping of rpoD implicates the major sigma factor of Bacillus subtilis RNA polymerase in sporulation initiation. Mol Gen Genet. 1985;201(1):88–95. doi: 10.1007/BF00397991. [DOI] [PubMed] [Google Scholar]
- Ratnaningsih E., Dharmsthiti S., Krishnapillai V., Morgan A., Sinclair M., Holloway B. W. A combined physical and genetic map of Pseudomonas aeruginosa PAO. J Gen Microbiol. 1990 Dec;136(12):2351–2357. doi: 10.1099/00221287-136-12-2351. [DOI] [PubMed] [Google Scholar]
- Römling U., Grothues D., Bautsch W., Tümmler B. A physical genome map of Pseudomonas aeruginosa PAO. EMBO J. 1989 Dec 20;8(13):4081–4089. doi: 10.1002/j.1460-2075.1989.tb08592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römling U., Tümmler B. The impact of two-dimensional pulsed-field gel electrophoresis techniques for the consistent and complete mapping of bacterial genomes: refined physical map of Pseudomonas aeruginosa PAO. Nucleic Acids Res. 1991 Jun 25;19(12):3199–3206. doi: 10.1093/nar/19.12.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Stachelek C., Konigsberg W., Rupp W. D. Sequences of the recA gene and protein. Proc Natl Acad Sci U S A. 1980 May;77(5):2611–2615. doi: 10.1073/pnas.77.5.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Notani N. K., McCarthy D., Clayton N. L. Transformation of Haemophilus influenzae by plasmid RSF0885 containing a cloned segment of chromosomal deoxyribonucleic acid. J Bacteriol. 1981 Dec;148(3):804–811. doi: 10.1128/jb.148.3.804-811.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. L., Econome J. G., Schutt A., Klco S., Cantor C. R. A physical map of the Escherichia coli K12 genome. Science. 1987 Jun 12;236(4807):1448–1453. doi: 10.1126/science.3296194. [DOI] [PubMed] [Google Scholar]
- Taschke C., Klinkert M. Q., Wolters J., Herrmann R. Organization of the ribosomal RNA genes in Mycoplasma hyopneumoniae: the 5S rRNA gene is separated from the 16S and 23S rRNA genes. Mol Gen Genet. 1986 Dec;205(3):428–433. doi: 10.1007/BF00338078. [DOI] [PubMed] [Google Scholar]
- Taylor D. E., Garner R. S., Allan B. J. Characterization of tetracycline resistance plasmids from Campylobacter jejuni and Campylobacter coli. Antimicrob Agents Chemother. 1983 Dec;24(6):930–935. doi: 10.1128/aac.24.6.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Taylor D. E. Natural transformation in Campylobacter species. J Bacteriol. 1990 Feb;172(2):949–955. doi: 10.1128/jb.172.2.949-955.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzmann C. J., Cunningham P. R., Ofengand J. Cloning, in vitro transcription, and biological activity of Escherichia coli 23S ribosomal RNA. Nucleic Acids Res. 1990 Jun 25;18(12):3515–3520. doi: 10.1093/nar/18.12.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenman W. M., Chai J., Louie T. J., Goudreau C., Lior H., Newell D. G., Pearson A. D., Taylor D. E. Antigenic analysis of Campylobacter flagellar protein and other proteins. J Clin Microbiol. 1985 Jan;21(1):108–112. doi: 10.1128/jcm.21.1.108-112.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W., Chang N., Taylor D. E. Pulsed-field gel electrophoresis of Campylobacter jejuni and Campylobacter coli genomic DNA and its epidemiologic application. J Infect Dis. 1991 May;163(5):1068–1072. doi: 10.1093/infdis/163.5.1068. [DOI] [PubMed] [Google Scholar]
- Yan W., Taylor D. E. Characterization of erythromycin resistance in Campylobacter jejuni and Campylobacter coli. Antimicrob Agents Chemother. 1991 Oct;35(10):1989–1996. doi: 10.1128/aac.35.10.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W., Taylor D. E. Sizing and mapping of the genome of Campylobacter coli strain UA417R using pulsed-field gel electrophoresis. Gene. 1991 May 15;101(1):117–120. doi: 10.1016/0378-1119(91)90232-z. [DOI] [PubMed] [Google Scholar]
- von Meyenburg K., Jørgensen B. B., Nielsen J., Hansen F. G. Promoters of the atp operon coding for the membrane-bound ATP synthase of Escherichia coli mapped by Tn10 insertion mutations. Mol Gen Genet. 1982;188(2):240–248. doi: 10.1007/BF00332682. [DOI] [PubMed] [Google Scholar]