Abstract
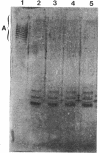
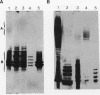
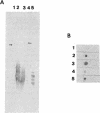
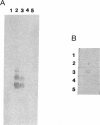
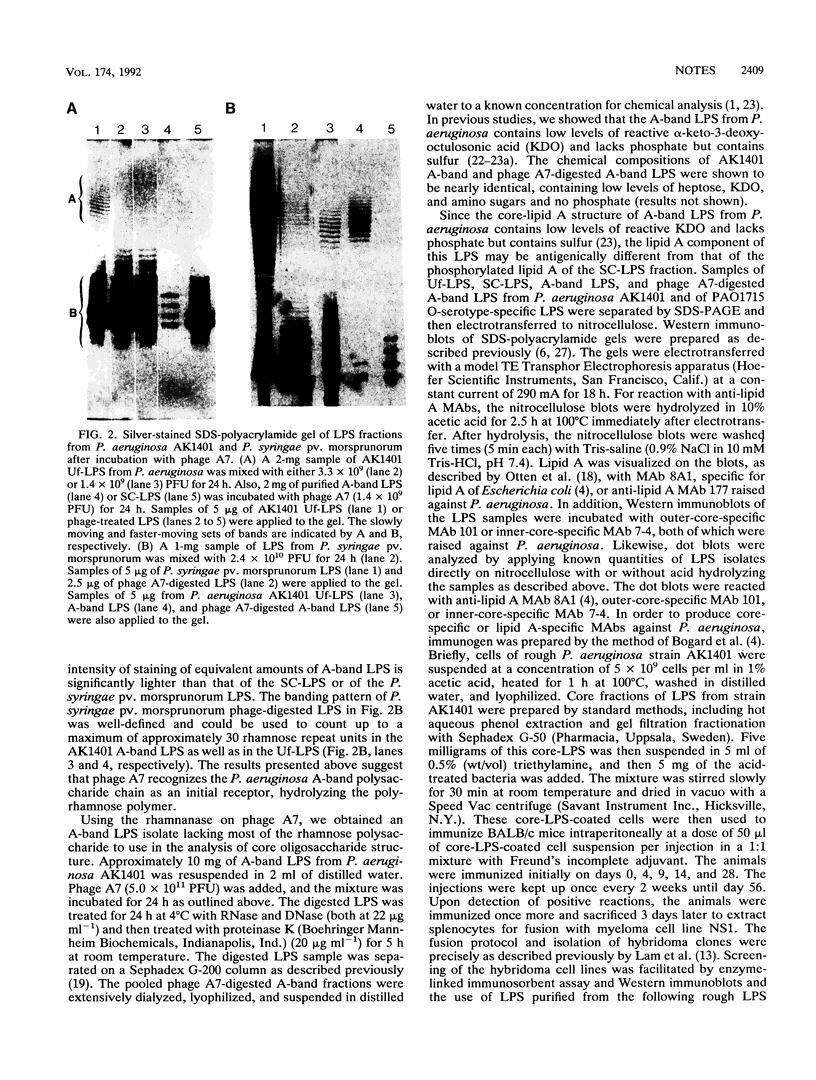
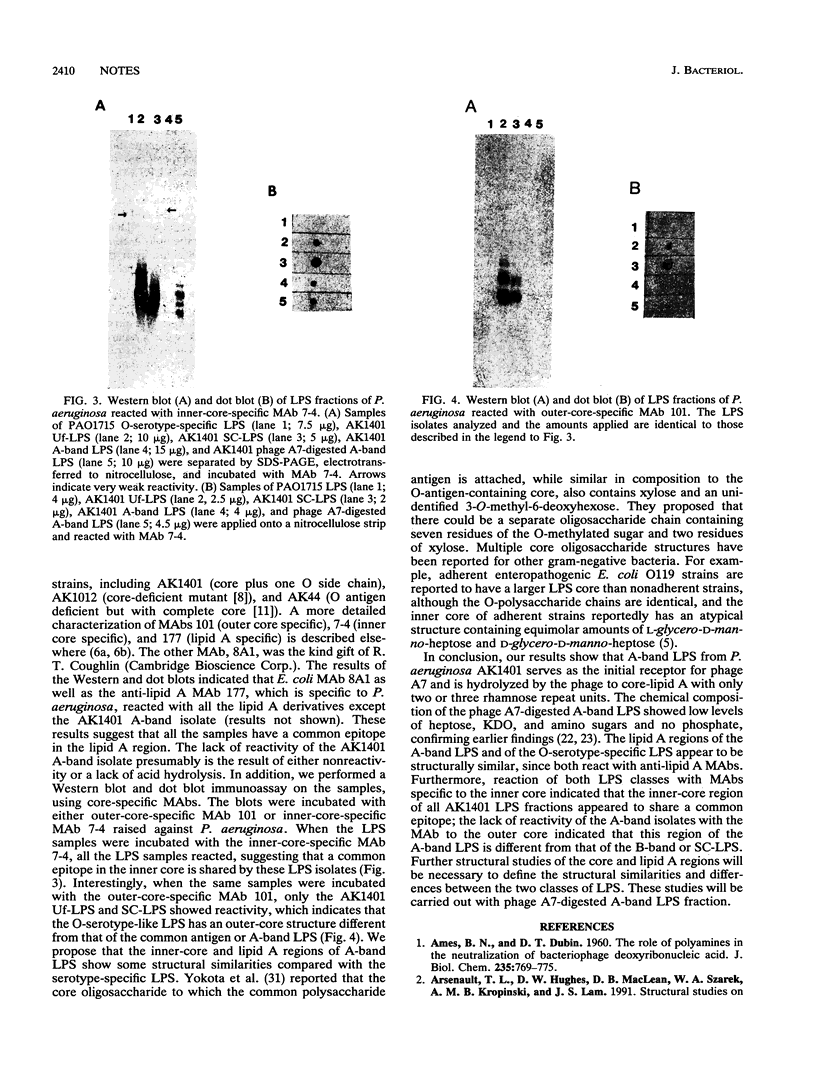
A-band, a D-rhamnose-containing common lipopolysaccharide antigen isolated from Pseudomonas aeruginosa AK1401, was found to be a receptor for bacteriophage A7. The phage-borne rhamnanase was capable of hydrolyzing the A-band to expose core-lipid A containing only two or three rhamnose repeats. Interaction of the hydrolyzed A-band with core- or lipid A-specific monoclonal antibodies revealed that common epitopes exist in the inner core and lipid A regions, while the outer core of A-band appears to be different from that of the serotype-specific (B-band) lipopolysaccharide.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Berry D., Kropinski A. M. Effect of lipopolysaccharide mutations and temperature on plasmid transformation efficiency in Pseudomonas aeruginosa. Can J Microbiol. 1986 May;32(5):436–438. doi: 10.1139/m86-082. [DOI] [PubMed] [Google Scholar]
- Bogard W. C., Jr, Dunn D. L., Abernethy K., Kilgarriff C., Kung P. C. Isolation and characterization of murine monoclonal antibodies specific for gram-negative bacterial lipopolysaccharide: association of cross-genus reactivity with lipid A specificity. Infect Immun. 1987 Apr;55(4):899–908. doi: 10.1128/iai.55.4.899-908.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. E., Anderson A. N., Perry M. B. Differences between the LPS cores in adherent and non-adherent strains of enteropathogenic Escherichia coli 0119. FEMS Microbiol Lett. 1991 May 1;64(1):13–17. doi: 10.1016/0378-1097(91)90201-k. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Dubray G., Bezard G. A highly sensitive periodic acid-silver stain for 1,2-diol groups of glycoproteins and polysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 15;119(2):325–329. doi: 10.1016/0003-2697(82)90593-0. [DOI] [PubMed] [Google Scholar]
- Jarrell K. F., Kropinski A. M. Isolation and characterization of a bacteriophage specific for the lipopolysaccharide of rough derivatives of Pseudomonas aeruginosa strain PAO. J Virol. 1981 May;38(2):529–538. doi: 10.1128/jvi.38.2.529-538.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knirel Y. A. Polysaccharide antigens of Pseudomonas aeruginosa. Crit Rev Microbiol. 1990;17(4):273–304. doi: 10.3109/10408419009105729. [DOI] [PubMed] [Google Scholar]
- Kocharova N. A., Knirel' Iu A., Kochetkov N. K., Stanislavskii E. S. Kharakteristika ramnana, vydelennogo iz preparatov lipopolisakharidov Pseudomonas aeruginosa. Bioorg Khim. 1988 May;14(5):701–703. [PubMed] [Google Scholar]
- Kropinski A. M., Jewell B., Kuzio J., Milazzo F., Berry D. Structure and functions of Pseudomonas aeruginosa lipopolysaccharide. Antibiot Chemother (1971) 1985;36:58–73. doi: 10.1159/000410472. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lam J. S., MacDonald L. A., Lam M. Y., Duchesne L. G., Southam G. G. Production and characterization of monoclonal antibodies against serotype strains of Pseudomonas aeruginosa. Infect Immun. 1987 May;55(5):1051–1057. doi: 10.1128/iai.55.5.1051-1057.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam M. Y., McGroarty E. J., Kropinski A. M., MacDonald L. A., Pedersen S. S., Høiby N., Lam J. S. Occurrence of a common lipopolysaccharide antigen in standard and clinical strains of Pseudomonas aeruginosa. J Clin Microbiol. 1989 May;27(5):962–967. doi: 10.1128/jcm.27.5.962-967.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGroarty E. J., Rivera M. Growth-dependent alterations in production of serotype-specific and common antigen lipopolysaccharides in Pseudomonas aeruginosa PAO1. Infect Immun. 1990 Apr;58(4):1030–1037. doi: 10.1128/iai.58.4.1030-1037.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlradt P. F., Golecki J. R. Asymmetrical distribution and artifactual reorientation of lipopolysaccharide in the outer membrane bilayer of Salmonella typhimurium. Eur J Biochem. 1975 Feb 21;51(2):343–352. doi: 10.1111/j.1432-1033.1975.tb03934.x. [DOI] [PubMed] [Google Scholar]
- Otten S., Iyer S., Johnson W., Montgomery R. Serospecific antigens of Legionella pneumophila. J Bacteriol. 1986 Sep;167(3):893–904. doi: 10.1128/jb.167.3.893-904.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson A. A., McGroarty E. J. High-molecular-weight components in lipopolysaccharides of Salmonella typhimurium, Salmonella minnesota, and Escherichia coli. J Bacteriol. 1985 May;162(2):738–745. doi: 10.1128/jb.162.2.738-745.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk A. V., Sletten A., Hignett R. C. Properties of phage-receptor lipopolysaccharide from Pseudomonas morsprunorum. J Gen Microbiol. 1976 Oct;96(2):375–381. doi: 10.1099/00221287-96-2-375. [DOI] [PubMed] [Google Scholar]
- Raetz C. R. Biochemistry of endotoxins. Annu Rev Biochem. 1990;59:129–170. doi: 10.1146/annurev.bi.59.070190.001021. [DOI] [PubMed] [Google Scholar]
- Rivera M., Bryan L. E., Hancock R. E., McGroarty E. J. Heterogeneity of lipopolysaccharides from Pseudomonas aeruginosa: analysis of lipopolysaccharide chain length. J Bacteriol. 1988 Feb;170(2):512–521. doi: 10.1128/jb.170.2.512-521.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera M., McGroarty E. J. Analysis of a common-antigen lipopolysaccharide from Pseudomonas aeruginosa. J Bacteriol. 1989 Apr;171(4):2244–2248. doi: 10.1128/jb.171.4.2244-2248.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada S., Kawamura T., Masuho Y., Tomibe K. A new common polysaccharide antigen of strains of Pseudomonas aeruginosa detected with a monoclonal antibody. J Infect Dis. 1985 Dec;152(6):1290–1299. doi: 10.1093/infdis/152.6.1290. [DOI] [PubMed] [Google Scholar]
- Smith A. R., Zamze S. E., Munro S. M., Carter K. J., Hignett R. C. Structure of the sidechain of lipopolysaccharide from Pseudomonas syringae pv. morsprunorum C28. Eur J Biochem. 1985 May 15;149(1):73–78. doi: 10.1111/j.1432-1033.1985.tb08895.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S., Kaya S., Araki Y., Ito E., Kawamura T., Sawada S. Occurrence of D-rhamnan as the common antigen reactive against monoclonal antibody E87 in Pseudomonas aeruginosa IFO 3080 and other strains. J Bacteriol. 1990 Oct;172(10):6162–6164. doi: 10.1128/jb.172.10.6162-6164.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S., Kaya S., Sawada S., Kawamura T., Araki Y., Ito E. Characterization of a polysaccharide component of lipopolysaccharide from Pseudomonas aeruginosa IID 1008 (ATCC 27584) as D-rhamnan. Eur J Biochem. 1987 Sep 1;167(2):203–209. doi: 10.1111/j.1432-1033.1987.tb13324.x. [DOI] [PubMed] [Google Scholar]