Abstract
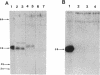
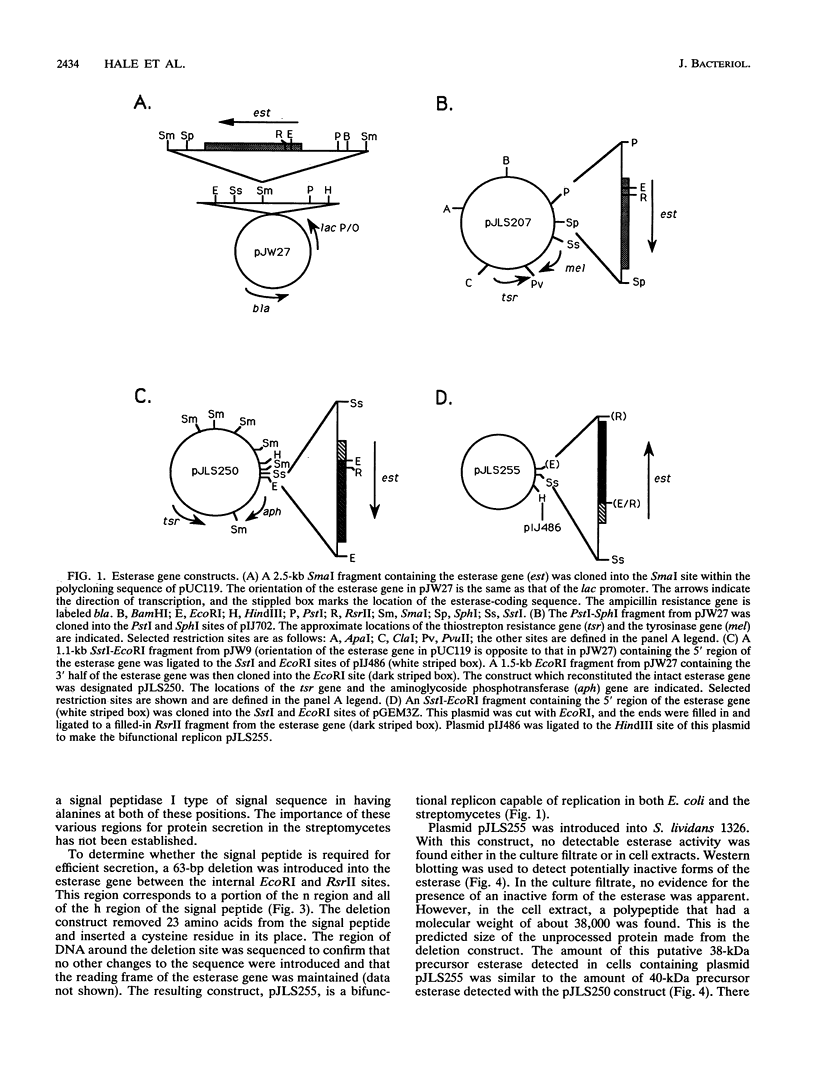
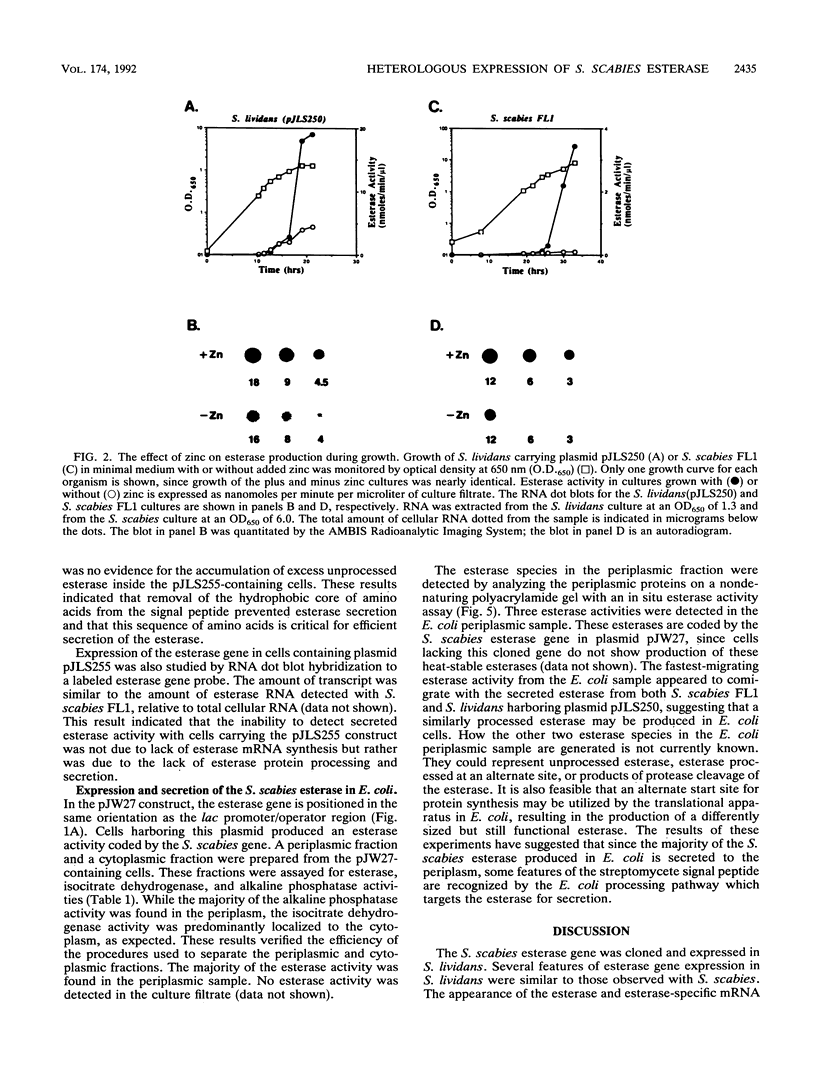
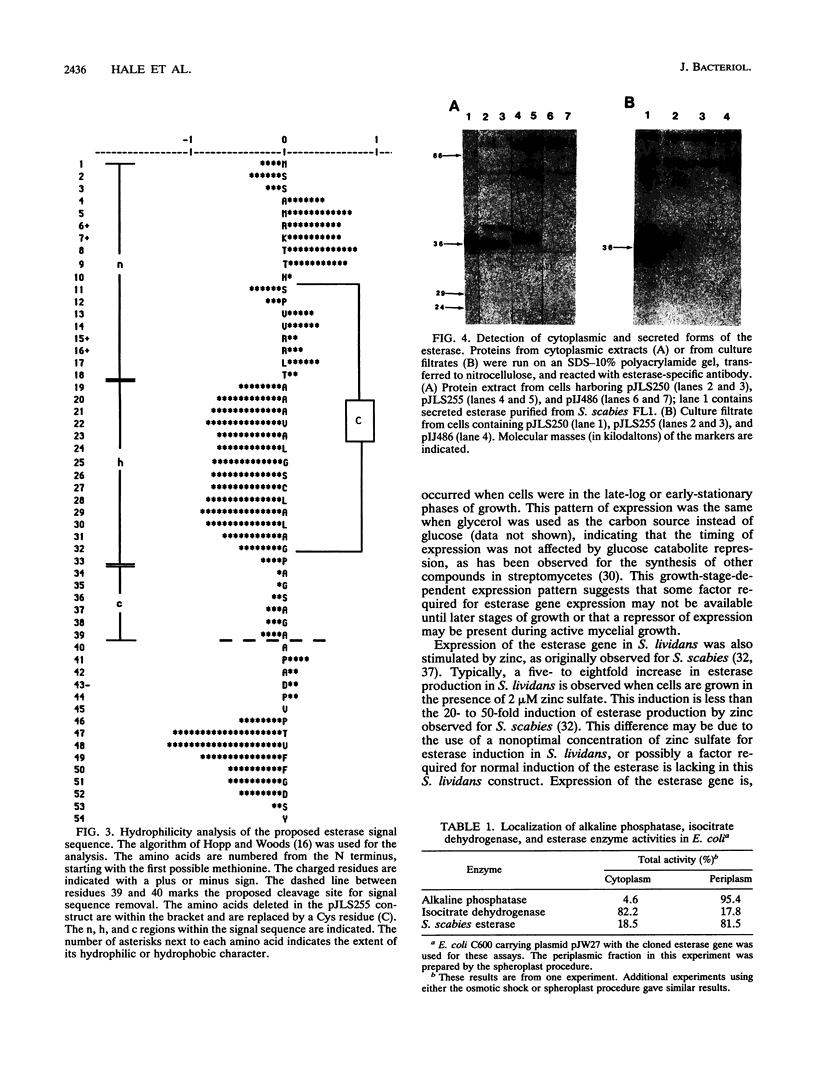
The esterase gene from Streptomyces scabies FL1 was cloned and expressed in Streptomyces lividans on plasmids pIJ486 and pIJ702. In S. lividans, the esterase gene was expressed during later stages of growth and was regulated by zinc, as is seen with S. scabies. The 36-kDa secreted form of the esterase was purified from S. lividans. N-terminal amino acid sequencing indicated that the processing site utilized in S. lividans for the removal of the signal sequence was the same as that recognized for processing in S. scabies. Western blots (immunoblots) revealed the presence of a 40-kDa precursor form of the esterase in cytoplasmic extracts. A 23-amino-acid deletion was introduced into the putative signal sequence for the esterase. When this deleted form of the esterase was expressed in S. lividans, a cytoplasmic 38-kDa precursor protein was produced but no secreted esterase was detected, suggesting the importance of the deleted sequence for efficient processing and secretion. The esterase gene was also cloned into the pUC119 plasmid in Escherichia coli. By using the lac promoter sequence, the esterase gene was expressed, and the majority of the esterase was localized to the periplasmic space.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender E., Koller K. P., Engels J. W. Secretory synthesis of human interleukin-2 by Streptomyces lividans. Gene. 1990 Feb 14;86(2):227–232. doi: 10.1016/0378-1119(90)90283-w. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Jones G. H., Joseph R., Buttner M. J., Ward J. M. The agarase gene (dag A) of Streptomyces coelicolor A3(2): affinity purification and characterization of the cloned gene product. J Gen Microbiol. 1987 Aug;133(8):2089–2096. doi: 10.1099/00221287-133-8-2089. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chang P. C., Kuo T. C., Tsugita A., Lee Y. H. Extracellular metalloprotease gene of Streptomyces cacaoi: structure, nucleotide sequence and characterization of the cloned gene product. Gene. 1990 Mar 30;88(1):87–95. doi: 10.1016/0378-1119(90)90063-w. [DOI] [PubMed] [Google Scholar]
- Doran J. L., Leskiw B. K., Aippersbach S., Jensen S. E. Isolation and characterization of a beta-lactamase-inhibitory protein from Streptomyces clavuligerus and cloning and analysis of the corresponding gene. J Bacteriol. 1990 Sep;172(9):4909–4918. doi: 10.1128/jb.172.9.4909-4918.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duez C., Piron-Fraipont C., Joris B., Dusart J., Urdea M. S., Martial J. A., Frère J. M., Ghuysen J. M. Primary structure of the Streptomyces R61 extracellular DD-peptidase. 1. Cloning into Streptomyces lividans and nucleotide sequence of the gene. Eur J Biochem. 1987 Feb 2;162(3):509–518. doi: 10.1111/j.1432-1033.1987.tb10669.x. [DOI] [PubMed] [Google Scholar]
- Eckhardt T., Strickler J., Gorniak L., Burnett W. V., Fare L. R. Characterization of the promoter, signal sequence, and amino terminus of a secreted beta-galactosidase from "Streptomyces lividans". J Bacteriol. 1987 Sep;169(9):4249–4256. doi: 10.1128/jb.169.9.4249-4256.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Henderson G., Krygsman P., Liu C. J., Davey C. C., Malek L. T. Characterization and structure of genes for proteases A and B from Streptomyces griseus. J Bacteriol. 1987 Aug;169(8):3778–3784. doi: 10.1128/jb.169.8.3778-3784.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood D. A., Kieser T., Wright H. M., Bibb M. J. Plasmids, recombination and chromosome mapping in Streptomyces lividans 66. J Gen Microbiol. 1983 Jul;129(7):2257–2269. doi: 10.1099/00221287-129-7-2257. [DOI] [PubMed] [Google Scholar]
- Hoshiko S., Makabe O., Nojiri C., Katsumata K., Satoh E., Nagaoka K. Molecular cloning and characterization of the Streptomyces hygroscopicus alpha-amylase gene. J Bacteriol. 1987 Mar;169(3):1029–1036. doi: 10.1128/jb.169.3.1029-1036.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T., LaPorte D. C. Isocitrate dehydrogenase kinase/phosphatase: aceK alleles that express kinase but not phosphatase activity. J Bacteriol. 1991 Mar;173(5):1801–1806. doi: 10.1128/jb.173.5.1801-1806.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz E., Thompson C. J., Hopwood D. A. Cloning and expression of the tyrosinase gene from Streptomyces antibioticus in Streptomyces lividans. J Gen Microbiol. 1983 Sep;129(9):2703–2714. doi: 10.1099/00221287-129-9-2703. [DOI] [PubMed] [Google Scholar]
- Kieser T., Hopwood D. A., Wright H. M., Thompson C. J. pIJ101, a multi-copy broad host-range Streptomyces plasmid: functional analysis and development of DNA cloning vectors. Mol Gen Genet. 1982;185(2):223–228. doi: 10.1007/BF00330791. [DOI] [PubMed] [Google Scholar]
- Kolattukudy P. E. Biopolyester membranes of plants: cutin and suberin. Science. 1980 May 30;208(4447):990–1000. doi: 10.1126/science.208.4447.990. [DOI] [PubMed] [Google Scholar]
- Koller K. P., Riess G. Heterologous expression of the alpha-amylase inhibitor gene cloned from an amplified genomic sequence of Streptomyces tendae. J Bacteriol. 1989 Sep;171(9):4953–4957. doi: 10.1128/jb.171.9.4953-4957.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984 Dec;10(3-4):203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lichenstein H., Brawner M. E., Miles L. M., Meyers C. A., Young P. R., Simon P. L., Eckhardt T. Secretion of interleukin-1 beta and Escherichia coli galactokinase by Streptomyces lividans. J Bacteriol. 1988 Sep;170(9):3924–3929. doi: 10.1128/jb.170.9.3924-3929.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. F., Daza A., Vigal T., Alegre T., Garcia M. D., Liras P., Gil J. A. Cloning of amylase and alkaline phosphatase genes from Streptomyces griseus as secretion vectors. Biochem Soc Trans. 1989 Apr;17(2):342–344. doi: 10.1042/bst0170342. [DOI] [PubMed] [Google Scholar]
- Martin J. F., Demain A. L. Control of antibiotic biosynthesis. Microbiol Rev. 1980 Jun;44(2):230–251. doi: 10.1128/mr.44.2.230-251.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen D. A., Schottel J. L. Purification and characterization of a novel extracellular esterase from pathogenic Streptomyces scabies that is inducible by zinc. J Bacteriol. 1987 May;169(5):1967–1971. doi: 10.1128/jb.169.5.1967-1971.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaso H., Saito S., Saito H., Takahashi H. Nucleotide sequence and expression of a Streptomyces griseosporeus proteinaceous alpha-amylase inhibitor (HaimII) gene. J Bacteriol. 1988 Oct;170(10):4451–4457. doi: 10.1128/jb.170.10.4451-4457.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piron-Fraipont C., Lenzini M. V., Dusart J., Ghuysen J. M. Transcriptional analysis of the DD-peptidase/penicillin-binding protein-encoding dac gene of Streptomyces R61: use of the promoter and signal sequences in a secretion vector. Mol Gen Genet. 1990 Aug;223(1):114–120. doi: 10.1007/BF00315803. [DOI] [PubMed] [Google Scholar]
- Raymer G., Willard J. M., Schottel J. L. Cloning, sequencing, and regulation of expression of an extracellular esterase gene from the plant pathogen Streptomyces scabies. J Bacteriol. 1990 Dec;172(12):7020–7026. doi: 10.1128/jb.172.12.7020-7026.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins P. W., Albright C., Benfield B. Cloning and expression of a Streptomyces plicatus chitinase (chitinase-63) in Escherichia coli. J Biol Chem. 1988 Jan 5;263(1):443–447. [PubMed] [Google Scholar]
- Robbins P. W., Trimble R. B., Wirth D. F., Hering C., Maley F., Maley G. F., Das R., Gibson B. W., Royal N., Biemann K. Primary structure of the Streptomyces enzyme endo-beta-N-acetylglucosaminidase H. J Biol Chem. 1984 Jun 25;259(12):7577–7583. [PubMed] [Google Scholar]
- Rosenberg M., Roegner V., Becker F. F. The quantitation of rat serum esterases by densitometry of acrylamide gels stained for enzyme activity. Anal Biochem. 1975 May 26;66(1):206–212. doi: 10.1016/0003-2697(75)90738-1. [DOI] [PubMed] [Google Scholar]
- Salinovich O., Montelaro R. C. Reversible staining and peptide mapping of proteins transferred to nitrocellulose after separation by sodium dodecylsulfate-polyacrylamide gel electrophoresis. Anal Biochem. 1986 Aug 1;156(2):341–347. doi: 10.1016/0003-2697(86)90263-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy T. J., Benson S. A., Emr S. D. Mechanisms of protein localization. Microbiol Rev. 1983 Sep;47(3):313–344. doi: 10.1128/mr.47.3.313-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumbly R. J., Robbins P. W., Belfort M., Ziegler F. D., Maley F., Trimble R. B. Amplified expression of streptomyces endo-beta-N-acetylglucosaminidase H in Escherichia coli and characterization of the enzyme product. J Biol Chem. 1985 May 10;260(9):5683–5690. [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Vértesy L., Tripier D., Koller K. P., Riess G. Disulphide bridge formation of proinsulin fusion proteins during secretion in Streptomyces. Biol Chem Hoppe Seyler. 1991 Mar;372(3):187–192. doi: 10.1515/bchm3.1991.372.1.187. [DOI] [PubMed] [Google Scholar]
- Walsh K. A., Ericsson L. H., Parmelee D. C., Titani K. Advances in protein sequencing. Annu Rev Biochem. 1981;50:261–284. doi: 10.1146/annurev.bi.50.070181.001401. [DOI] [PubMed] [Google Scholar]
- Ward J. M., Janssen G. R., Kieser T., Bibb M. J., Buttner M. J., Bibb M. J. Construction and characterisation of a series of multi-copy promoter-probe plasmid vectors for Streptomyces using the aminoglycoside phosphotransferase gene from Tn5 as indicator. Mol Gen Genet. 1986 Jun;203(3):468–478. doi: 10.1007/BF00422072. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G., Abrahmsén L. Species-specific variation in signal peptide design. Implications for protein secretion in foreign hosts. FEBS Lett. 1989 Feb 27;244(2):439–446. doi: 10.1016/0014-5793(89)80579-4. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]