Abstract
Mice infected with the protozoan parasite Trypanosoma brucei brucei and treated subcuratively with the trypanocidal drug diminazene aceturate develop an acute inflammatory meningoencephalitis with associated astrocytic proliferation. This reaction is very similar to that seen in the fatal posttreatment reactive encephalopathies that can occur in human African trypanosomiasis. The 11-amino acid neuropeptide substance P (SP) has recently been identified as a mediator in many inflammatory responses, and the development of potent, highly specific, nonpeptide SP antagonists has provided a new opportunity to investigate the possible involvement of SP in a variety of pathological conditions. We therefore postulated that SP may play a role in the development of the posttreatment inflammatory encephalopathy found in this experimental mouse model of African trypanosomiasis. In the present study RP-67,580, a SP antagonist that binds specifically to NK-1 receptors, was given intraperitoneally at a dose of 2 mg/kg twice daily to mice in which a severe meningoencephalitis had been produced. A significant reduction in both the severity of the inflammatory response (P = 0.0001) as well as the degree of astrocyte activation (P < 0.001) was found in the brains of these animals as compared with control mice that had not received RP-67,580. An inactive enantiomer of this SP antagonist, RP-68,651, had no effect on the central nervous system inflammatory reaction. We conclude from these findings that the neuropeptide SP plays a key role in the development of the severe central nervous system inflammatory response associated with African trypanosomiasis.
Human African trypanosomiasis, or sleeping sickness, is caused by infection with the protozoan parasites Trypanosoma brucei gambiense or Trypanosoma brucei rhodesiense. Without chemotherapeutic intervention the condition is invariably fatal. Melarsoprol, a trivalent arsenical derivative, is the drug of choice for the treatment of late-stage human sleeping sickness, but its use can lead to the development of a posttreatment reactive encephalopathy (PTRE) which can prove fatal in up to 10% of patients (1, 2).
Pathologically, the central nervous system (CNS) lesions found in late-stage sleeping sickness are characterized by cellular infiltrates and perivascular cuffs composed mostly of macrophages, lymphocytes, and plasma cells, some of which may be morular, Russell body containing plasma cells and the PTRE is associated with an exacerbation of these changes (1, 3). The pathogenesis of CNS trypanosomiasis and of the posttreatment reaction is unclear. Possible explanations have included the release of parasite antigens within the CNS as a consequence of chemotherapy (4), immune complex deposition (5), or autoimmunity (6). An immune mediated mechanism seems likely since the nonsteroidal anti-inflammatory drug azathioprine can be used to prevent the development of the posttreatment reaction in a Trypanosoma brucei brucei mouse model (7).
This murine model, developed in our laboratory, mirrors the pathological changes seen in the CNS of patients with sleeping sickness. When mice are infected with T. b. brucei, they develop a chronic infection with parasites established in the CNS by day 21. In these untreated animals, histological changes in the CNS become apparent toward the terminal stages of infection. However, the reaction can be exacerbated if the mice are treated subcuratively on or after day 21 of infection with a wide variety of trypanocidal compounds—e.g., diminazene aceturate or melarsoprol. Animals treated in this manner clear the trypanosomes from the extravascular compartments but not the CNS (8). This results in the rapid development of a posttreatment meningoencephalitis, and these subcuratively treated mice eventually relapse with parasitaemia.
Transcripts for a variety of cytokines that can initiate inflammation, such as tumor necrosis factor α, macrophage inflammatory protein 1 (MIP-1), and interleukin 1α (IL-1α), have been detected within the CNS of these animals (9). Transcripts for tumor necrosis factor-α, IL-6, interferon-γ, and MIP-1 have also been detected before any inflammatory processes are evident and coinciding with the appearance of activated astrocytes (10), which are capable of producing many of these cytokines (11, 12). Previous studies (10) have indicated that astrocyte activation is an early event during the PTRE and may play an important role in determining the pathology associated with the condition.
Recent evidence indicates that substance P (SP) may be involved in immune responses and inflammation (13). SP is an 11-amino acid neuropeptide that is widely distributed in the central and peripheral nervous systems and has a well-established role as a neurotransmitter. SP stimulates peripheral blood monocytes to produce IL-1, tumor necrosis factor, and IL-6 (14–17) and directly stimulates T cell proliferation (18) and antibody production by B cells (19, 20). Increased numbers of SP receptors are found at sites of peripheral inflammatory lesions (21), and recent data suggest an increase in the number of SP receptors around lesions in the CNS (22). Astrocytes can express receptors for SP (23, 24) and SP has been shown to induce IL-1 (25) and IL-6 (26) secretion by astrocytes and to enhance the secretion of tumor necrosis factor-α from neuroglial cells stimulated with lipopolysaccharide (24).
These data led us to postulate that SP may be involved in the induction of the trypanosome-induced meningoencephalitis. Highly specific nonpeptide SP antagonists have recently been developed that provide an ideal means to study the involvement of SP in such conditions. One of these antagonists (CP-96,345) has been shown to markedly reduce the inflammatory response to an allograft in rats in vivo and to inhibit the intestinal response of rat ileum exposed to Clostridium difficile toxin A (27). We report here the effects of RP-67,580, a SP antagonist that binds specifically to NK-1 receptors (28), on the meningoencephalopathy associated with T. b. brucei infection in our mouse model.
MATERIALS AND METHODS
RP-67,580 and RP-68,651.
The SP antagonist RP-67,580 {2-[1-imino-2-(2-methoxyphenyl)ethyl]-7,7-diphenyl-4-perhydroisoindolone (3aR, 7aR)} and its inactive enantiomer RP-68,651 {3aS,7aS} were provided by Rhône-Poulenc Rorer (Vitry sur Eine, France). These compounds were dissolved in isotonic saline at a concentration of 0.875 mg/ml before use.
Animals, Infections and Treatments.
Female CD-1 mice (Charles River Breeding Laboratories) of 28–35 g body weight were infected intraperitoneally (i.p) with 4 × 104 parasites of T. b. brucei (cloned stabilate GVR 35/C1.5). These mice were treated on day 28 postinfection with diminazene aceturate (Berenil; Hoechst Pharmaceuticals) (40 mg/kg, i.p.) to induce a posttreatment reaction. Seven days later the animals were given RP-67,580 (2 mg/kg i.p.) twice daily for 10 days and killed at the end this period. This protocol was carried out on three separate occasions using a total of 26 mice. A slightly modified fourth experiment was also performed. In this regimen 9 mice were infected and diminazene aceturate treated as before. However, in this experiment the SP antagonist treatment was initiated on day 27 postinfection, 1 day before the diminazene aceturate treatment, and continued for 21 days at which time the animals were killed.
In parallel with the above experiments various control regimens were followed. These protocols were as follows: infected mice given diminazene aceturate but no RP-67,580; infected, diminazene aceturate-treated mice given RP-68,651, an inactive enantiomer of RP-67,580, in place of the SP antagonist; and uninfected mice given the same drug regimens as the infected animals.
Mice from all infected groups were retained for general parasitological monitoring to assess when relapse of parasitaemia occurred after diminazene aceturate treatment.
Histopathology.
At necropsy the brains were removed, fixed in neutral buffered formalin, and paraffin wax embedded. Sections were cut at a thickness of 3 μm and stained with haematoxylin/eosin for light microscopy. The severity of neuropathology was graded in a blinded fashion by two individuals using parameters detailed in Table 1. Using this system the severity of the inflammatory reaction in the tissue could be graded on a scale of 0–4, where 0 indicated a normal histology and grade 4 indicated a severe meningoencephalitis. The assigning of a numerical value to a particular severity of inflammation allows the inflammatory reaction to be assessed statistically. The results were analyzed using a randomized block design analysis of variance and P values calculated for the combined results of the initial three experiments alone and in combination with the results from the modified fourth experiment. P values of less than 0.05 were considered to be significant.
Table 1.
Parameters defining the injury score allocated to the serverity of neuropathology
| Score
|
|||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| Meningitis | None | Mild | Moderate | Severe | Severe |
| Perivascular cuffing | None | None | Mild cuffing of some vessels | Prominent cuffing of some vessels | Prominent cuffing of most vessels |
| Encephalitis as defined by cellular activity in the neuropil | None | None | None | Moderate | Severe |
Injury scores are given horizontally; the parameters use are shown vertically.
Astrocyte activation was assessed by an indirect immunocytochemistry technique to identify glial fibrillary acidic protein (GFAP), a major protein of glial intermediate filaments in differentiated astrocytes. All antibodies were purchased from Dako. The intensity and degree of staining with GFAP, the number of stained cells, the topographical distribution, as well as the complexity of the astrocytic cytoplasmic processes were the criteria used to assess levels of activation compared with controls. The number of astrocytes present was assessed, using a graticule, in a representative experiment by counting five random fields in the hippocampal area. The brain sections of 8 mice from each treatment regimen were assessed, each section was counted twice to give a total of 80 fields in each group. As before a P value of less than 0.05 was considered significant.
RESULTS
Effect of the SP Antagonist RP-67,580 on Trypanosome-Induced Meningoencephalitis in Mouse Brain.
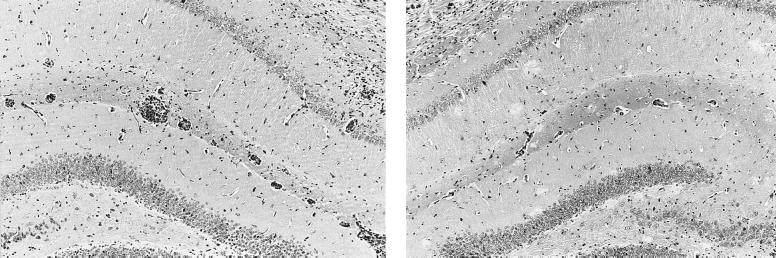
The results are summarized in Table 2. Histological examination of brain tissue from mice exposed to parasite showed, on average, a severe meningitis, with prominent perivascular cuffing of most vessels and a moderate encephalitis as defined by cellular activity in the neuropil (Fig. 1). The inflammatory cells were comprised mainly of lymphocytes, plasma cells, and macrophages. This inflammatory reaction was markedly reduced in mice that were treated with RP-67,580. These animals exhibited a moderate meningitis with perivascular cuffing of some vessels and no encephalitis (Fig. 1). Statistical analysis of the combined results from the initial three experiments showed a P value of 0.0002, a probability of 1 in 5000 that the reduction in the severity of the pathology was due to chance alone. If the results from the fourth experiment are included in the analysis the significance level is raised to P = 0.0001.
Table 2.
Effect of SP antagonist RP-67,580 and enantiomer RP-68,651 on trypanosome-induced meningoencephalitis
| Treatment regimen | Neuropathological grading |
|---|---|
| Trypanosome + salinea (n = 25) | 3.3 ± 0.17 |
| Trypanosome + RP-67,580b (n = 26) | 2.4 ± 0.18 |
| Trypanosome + RP-67,580c (n = 35) | 2.4 ± 0.17 |
| Trypanosome + RP-68,651d (n = 5) | 3.5 ± 0.22 |
Brain tissue from trypanosome infected animals exposed to saline or RP-67,580 treatment were processed as described. The severity of the neuropathology was graded by a score of 0–4. Data are expressed as means ± SE for each group. n, Number of mice tested; a, saline control; b, combined results of initial three experiments; c, combined results of all four experiments; d, enantiomer control. a vs. b, P = 0.0002; a vs. c, P = 0.0001; a vs. d, P = 0.38.
Figure 1.
Coronal, haematoxylin/eosin-stained section through hippocampal brain region from a T. b. brucei-infected mouse after subcurative therapy with diminazene aceturate (Left). Section from a similarly treated mouse also give RP-67,580 (Right). Note the marked reduction in the level of inflammation present in the SP antagonist-treated animal. (×100.)
Effect of the SP Antagonist RP-67,580 on Astrocyte Activation.
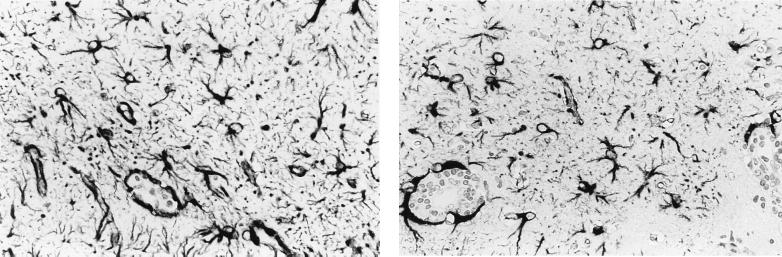
Infected diminazene aceturate treated animals showed a diffuse astrocyte activation throughout the cerebral and cerebellar cortices. Large reactive astrocytes with many long elaborately branched processes were evident, particularly in areas of high inflammation. Infected, diminazene aceturate-treated animals given RP-67,580 showed a reduction in this astrocyte activation (Fig. 2). In these mice the astrocytes were fewer in number and stained more weakly, there was a reduction in the intricacy of stellation, and their distribution was limited to the corpus callosum, along the choroid fissure and around the ventricles. Statistical analysis of the representative experiment showed that the reduction of GFAP-positive cells in RP-67,580-treated mouse brain compared with brains from untreated infected mice was significant (P < 0.001). Results are summarized in Table 3.
Figure 2.
Coronal, GFAP-stained section through the hippocampal brain region from a T. b. brucei-infected mouse after subcurative treatment with diminazene aceturate (Left). Section from a similarly treated mouse also given RP-67,580 (Right). Note the reduction in the intensity of staining, degree of stellation and number of stained cells present. (×400.)
Table 3.
Effect of SP antagonist RP-67,580 on the number of astrocytes found in trypanosome-infected mouse brain
| Treatment regimen | No. of astrocytes |
|---|---|
| Trypanosome + salinea (n = 80) | 187 ± 3 |
| Trypanosome + RP-67,580b (n = 80) | 102 ± 2 |
Brain tissue from trypanosome infected animals exposed to saline or RP-67,580 treatment were stained for GFAP as described. The number of astrocytes was assessed using a graticule, a total of 80 random fields per group were counted. Data are expressd as mean ± SE for each group. n, Number of fields assessed. a vs. b, P < 0.001.
The Effect of the Enantiomer RP-68,651.
The enantiomer RP-68,651 had no effect on the meningoencephalitis induced by subcurative chemotherapy in our T. b. brucei-infected mouse model. A similar pathological picture to that shown in Fig. 1 Left was found.
Uninfected Animals.
There were no pathological changes found in any of the uninfected animals given the same treatment regimens as the infected animals (data not shown).
DISCUSSION
Our data show that treatment with an antagonist to SP (RP-67,580) ameliorates an established meningoencephalitis following trypanocidal chemotherapy in mice, clearly implicating the involvement of a SP-dependent pathway in the generation of this response. As far as we are aware, the use of such a neuropeptide antagonist to diminish an inflammatory response in the CNS has not previously been demonstrated.
While the exact site of action of the SP antagonist in the present study has yet to be determined, there are several possibilities. SP may be released from multiple cell types within the brain including astrocytes, neurons, resident and circulating lymphocytes, as well as macrophages and microglia. The small number of neutrophils that migrate into the CNS during inflammation in response to T. b. brucei infection may be an additional source of SP. Further multiple cell types in the brain can express NK-1 receptors and may therefore be potential site of action for RP-67,580. An interaction between the SP antagonist and astrocytes, however, is strongly suggested by the observation that this cell type has specific receptors for SP (29), and also by the reduction in of the degree of astrocyte activation as assessed by GFAP immunoreactivity in this study. Further, SP immunoreactive astrocytes have been described within the glial scars in multiple sclerosis brain tissue (24, 30), and a positive feedback loop may develop to augment the inflammatory reaction in that condition. However, it cannot be ascertained from our study whether the SP antagonist is acting directly on astrocytes or indirectly by reducing their activation through prevention of stimulatory cytokine production by other cells that express SP receptors such as lymphocytes, macrophages, and neurons. It is also possible that SP may have a direct action on endothelial cells (31), possibly leading to increased vascular permeability. The presence of an antagonist might reduce this permeability and therefore limit the extent of any inflammatory response.
Neither the exact mechanism by which SP modulates inflammation nor its site of action can be ascertained from the present study. Further experiments will be required to identify the specific cell types involved in SP-controlled inflammatory responses as well as their possible interactions and the kinetics of such reactions. Our findings raise the possibility that SP plays a role in generating the inflammatory changes seen in other neurological conditions in which prominent inflammatory changes occur such as HIV encephalitis. In both the latter disease and the T. b. brucei posttreatment meningoencephalitis the inflammatory reaction is out of proportion to the small amounts of virus or parasite respectively detectable within the brain so that indirect pathogenic mechanisms involving cytokine release may be important. In view of the possible therapeutic implications for human diseases, studies investigating the mechanism of action of SP in the control of immune responses in the CNS should be pursued.
Acknowledgments
We thank Barbara Bradley for technical assistance with animal infections, Iain McMillan and staff of the histology unit of the Department of Veterinary Pathology for tissue processing, and Dr. James Nicoll of the Department of Neuropathology, Southern General Hospital, Glasgow, for help and advice with regard to neuropathology. Assistance with statistical analyses from David Irvine is also gratefully acknowledged. This research was financially supported by the Wellcome Trust (reference no. 038131/Z/93/Z) and initiated by S.E.L. and P.G.E.K. while Fogarty International Scholars-in-Residence, National Institutes of Health, Bethesda, MD.
ABBREVIATIONS
- PTRE
posttreatment reactive encephalopathy
- CNS
central nervous system
- IL
interleukin
- SP
substance P
- GFAP
glial fibrillary acidic protein
References
- 1.Adams H, Doua A, Dago-Akribi A, Boa Y, Haller L. Neuropathol Appl Neurobiol. 1986;12:81–94. doi: 10.1111/j.1365-2990.1986.tb00682.x. [DOI] [PubMed] [Google Scholar]
- 2.Pepin J, Milord F. Trans R Soc Trop Med Hyg. 1991;85:222–224. doi: 10.1016/0035-9203(91)90032-t. [DOI] [PubMed] [Google Scholar]
- 3.Haller L, Adams H, Merowze F, Dago A. Am J Trop Med Hyg. 1986;5:94–99. doi: 10.4269/ajtmh.1986.35.94. [DOI] [PubMed] [Google Scholar]
- 4.Hunter C A, Jennings F W, Adams J H, Murray M, Kennedy P G E. Lancet. 1992;339:956–958. doi: 10.1016/0140-6736(92)91531-c. [DOI] [PubMed] [Google Scholar]
- 5.Lambert P H, Berney M, Kazyumba G L. J Clin Invest. 1981;181:77–85. doi: 10.1172/JCI110035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poltera A A. Trans R Soc Trop Med Hyg. 1980;74:706–715. doi: 10.1016/0035-9203(80)90183-2. [DOI] [PubMed] [Google Scholar]
- 7.Hunter C A, Jennigs F W, Kennedy P G E, Murray M. Neuropathol Appl Neurobiol. 1992;18:619–625. doi: 10.1111/j.1365-2990.1992.tb00833.x. [DOI] [PubMed] [Google Scholar]
- 8.Jennings F W, Gray G D. Contrib Microbiol Immunol. 1983;7:147–154. [PubMed] [Google Scholar]
- 9.Hunter C A, Gow J W, Jennings F W, Kennedy P G E, Murray M. Infect Immun. 1991;59:4636–4640. doi: 10.1128/iai.59.12.4636-4640.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunter C A, Jennings F W, Kennedy P G E, Murray M. Lab Invest. 1992;67:635–642. [PubMed] [Google Scholar]
- 11.Saukkonen K, Sande S, Cioffe C, Wolpe S, Sheery B, Cerami A, Tuomanen E. J Exp Med. 1990;171:439–448. doi: 10.1084/jem.171.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lieberman A P, Piyha P M, Shin H S, Shin M L. Proc Natl Acad Sci USA. 1989;86:6348–6352. doi: 10.1073/pnas.86.16.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGillis J P, Organist M L, Payan D G. FASEB J. 1987;46:196–199. [PubMed] [Google Scholar]
- 14.Lotz M, Vaughan J H, Carson D A. Science. 1988;241:1218–1221. doi: 10.1126/science.2457950. [DOI] [PubMed] [Google Scholar]
- 15.Lee H, Ho W, Douglas S D. Clin Diagn Lab Immunol. 1994;1:419–423. doi: 10.1128/cdli.1.4.419-423.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner F, Fink R, Hart R, Dancygier H. Regul Pept. 1987;19:355–364. doi: 10.1016/0167-0115(87)90177-7. [DOI] [PubMed] [Google Scholar]
- 17.Blum A M, Metwali A, Cook G, Mathew R C, Elliott D, Weinstock J V. J Immunol. 1993;151:225–233. [PubMed] [Google Scholar]
- 18.Payan D G, Brewster D R, Goetzl E J. J Immunol. 1983;131:1613–1615. [PubMed] [Google Scholar]
- 19.Blum A, Metwali A, Elliott D, Sandor M, Lynch R, Weinstock J V. J Neuroimmunol. 1996;66:1–10. doi: 10.1016/0165-5728(95)00163-8. [DOI] [PubMed] [Google Scholar]
- 20.Laurenzi M A, Persson M A A, Dalsgaard C-J, Ringden O. Scand J Immunol. 1989;30:695–701. doi: 10.1111/j.1365-3083.1989.tb02478.x. [DOI] [PubMed] [Google Scholar]
- 21.Mantyh C R, Troy S G, Zimmerman R P, Welto M L, Passaeo E P, Vigna S R, Maggio J E, Kruger L, Mantyh P. Proc Natl Acad Sci USA. 1988;85:3235–3239. doi: 10.1073/pnas.85.9.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mantyh P W, Johnson D J, Boehmer C G, Catton M D, Vinters H V, Maggio J E, Too H, Vigna S R. Proc Natl Acad Sci USA. 1989;86:5193–5197. doi: 10.1073/pnas.86.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torrens Y, Beaujouan J C, Saffroy M, Daguet de Montety M C, Bergstrom L, Glowinski J. Proc Natl Acad Sci USA. 1986;83:9216–9220. doi: 10.1073/pnas.83.23.9216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luber-Narod J, Kage R, Leeman S E. J Immunol. 1994;152:819–824. [PubMed] [Google Scholar]
- 25.Martin F C, Charles A C, Sanderson M J, Merrill J. Brain Res. 1992;599:13–18. doi: 10.1016/0006-8993(92)90846-2. [DOI] [PubMed] [Google Scholar]
- 26.Gitter B D, Regoli D, Howbert J J, Glasebrook A L, Waters D C. J Neuroimmunol. 1994;51:100–108. doi: 10.1016/0165-5728(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 27.Pothoulakis C, Castagliuolo I, LaMont J T, Jaffer A, O’Keane J C, Snider R M, Leeman S E. Proc Natl Acad Sci USA. 1994;91:947–951. doi: 10.1073/pnas.91.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Courteix C, Lavarenne J, Eschalier A. Eur J Pharamacol. 1993;241:267–270. doi: 10.1016/0014-2999(93)90213-2. [DOI] [PubMed] [Google Scholar]
- 29.Lin R C S. Clin Neurosci Neuropathol. 1995;7:310–312. [Google Scholar]
- 30.Barker R, Larner A. Med Hypotheses. 1992;37:40–43. doi: 10.1016/0306-9877(92)90011-z. [DOI] [PubMed] [Google Scholar]
- 31.Kostyk S K, Kowall N W, Hauser S L. Brain Res. 1989;504:284–288. doi: 10.1016/0006-8993(89)91369-3. [DOI] [PubMed] [Google Scholar]