Abstract
An extensive screening among microorganisms for the presence of post-proline-specific endopeptidase activity was performed. This activity was found among ordinary bacteria from soil samples but not among fungi and actinomycetes. This result is in contrast to the previous notion that this activity is confined to the genus Flavobacterium. A proline endopeptidase was isolated from a Xanthomonas sp. and characterized with respect to physicochemical and enzymatic properties. The enzyme is composed of a single peptide chain with a molecular weight of 75,000. The isoelectric point is 6.2. It is inhibited by diisopropylfluorophosphate and may therefore be classified as a serine endopeptidase. The activity profile is bell shaped with an optimum at pH 7.5. By using synthetic peptide substrates and intramolecular fluorescence quenching it was possible to study the influence of substrate structure on the rate of hydrolysis. The enzyme specifically hydrolyzed Pro-X peptide bonds. With Glu at position X, low rates of hydrolysis were observed; otherwise the enzyme exhibited little preference for particular amino acid residues at position X. A similar substrate preference was observed with respect to the amino acid residue preceding the prolyl residue in the substrate. The enzyme required a minimum of two amino acid residues toward the N terminus from the scissile bond, but further elongation of the peptide chain by up to six amino acid residues caused only a threefold increase in the rate of hydrolysis. Attempts to cleave at the prolyl residues in oxidized RNase failed, indicating that the enzyme does not hydrolyze long peptides, a peculiar property it shares with other proline-specific endopeptidases.
Full text
PDF

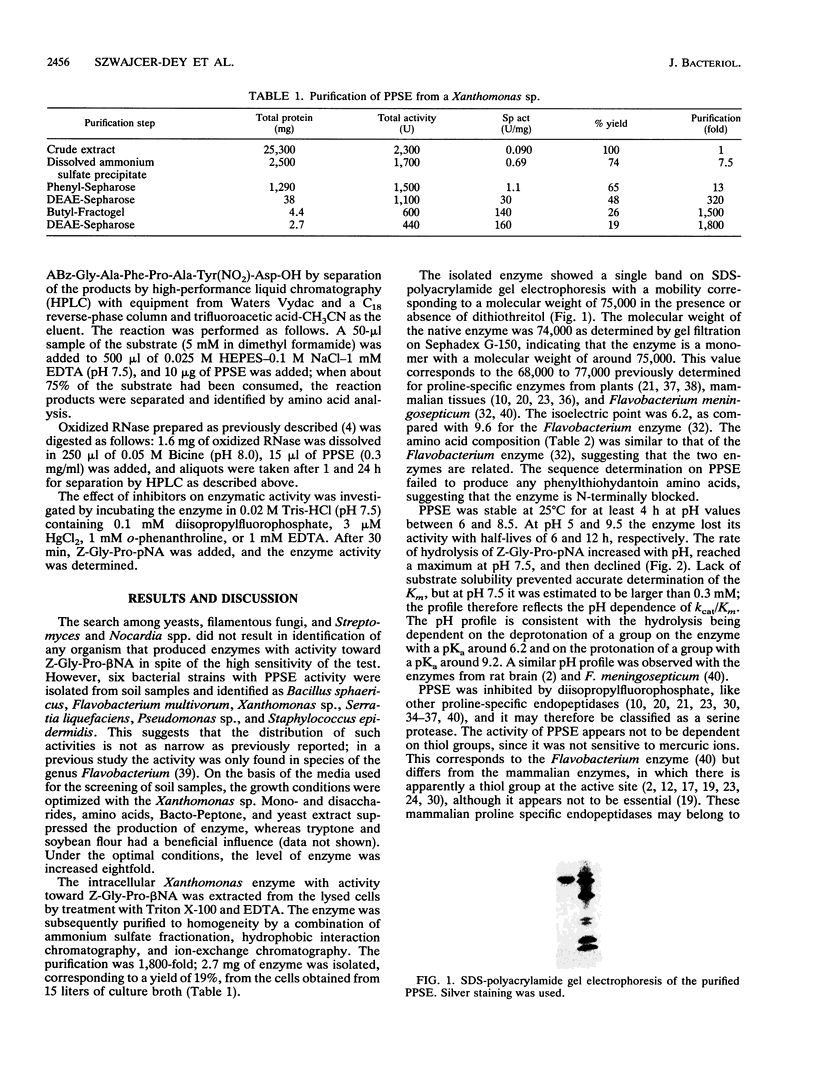
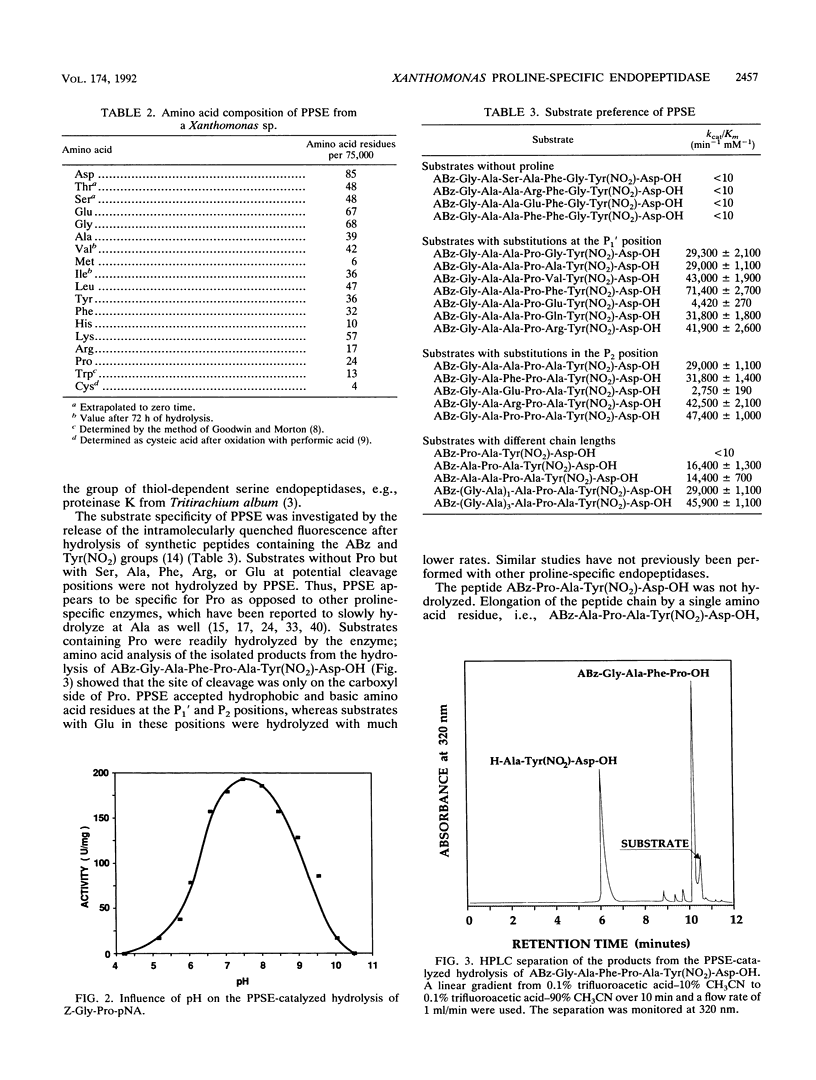


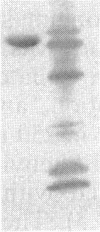
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbasi A., Voelter W., Zaidi Z. H. Isolation purification and properties of a site-specific proteolytic enzyme "valyl-proteinase" from Candida tropicalis. Biol Chem Hoppe Seyler. 1986 May;367(5):441–445. doi: 10.1515/bchm3.1986.367.1.441. [DOI] [PubMed] [Google Scholar]
- Andrews P. C., Hines C. M., Dixon J. E. Characterization of proline endopeptidase from rat brain. Biochemistry. 1980 Nov 25;19(24):5494–5500. doi: 10.1021/bi00565a005. [DOI] [PubMed] [Google Scholar]
- Betzel C., Pal G. P., Saenger W. Three-dimensional structure of proteinase K at 0.15-nm resolution. Eur J Biochem. 1988 Dec 1;178(1):155–171. doi: 10.1111/j.1432-1033.1988.tb14440.x. [DOI] [PubMed] [Google Scholar]
- Buttle D. J., Ritonja A., Pearl L. H., Turk V., Barrett A. J. Selective cleavage of glycyl bonds by papaya proteinase IV. FEBS Lett. 1990 Jan 29;260(2):195–197. doi: 10.1016/0014-5793(90)80101-n. [DOI] [PubMed] [Google Scholar]
- Drapeau G. R., Boily Y., Houmard J. Purification and properties of an extracellular protease of Staphylococcus aureus. J Biol Chem. 1972 Oct 25;247(20):6720–6726. [PubMed] [Google Scholar]
- Gilles A. M., Imhoff J. M., Keil B. alpha-Clostripain. Chemical characterization, activity, and thiol content of the highly active form of clostripain. J Biol Chem. 1979 Mar 10;254(5):1462–1468. [PubMed] [Google Scholar]
- Goodwin T. W., Morton R. A. The spectrophotometric determination of tyrosine and tryptophan in proteins. Biochem J. 1946;40(5-6):628–632. doi: 10.1042/bj0400628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalwant S., Porter A. G. Purification and characterization of human brain prolyl endopeptidase. Biochem J. 1991 May 15;276(Pt 1):237–244. doi: 10.1042/bj2760237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knisatschek H., Bauer K. Characterization of "thyroliberin-deamidating enzyme" as a post-proline-cleaving enzyme. Partial purification and enzyme-chemical analysis of the enzyme from anterior pituitary tissue. J Biol Chem. 1979 Nov 10;254(21):10936–10943. [PubMed] [Google Scholar]
- Meldal M., Breddam K. Anthranilamide and nitrotyrosine as a donor-acceptor pair in internally quenched fluorescent substrates for endopeptidases: multicolumn peptide synthesis of enzyme substrates for subtilisin Carlsberg and pepsin. Anal Biochem. 1991 May 15;195(1):141–147. doi: 10.1016/0003-2697(91)90309-h. [DOI] [PubMed] [Google Scholar]
- Moriyama A., Nakanishi M., Sasaki M. Porcine muscle prolyl endopeptidase and its endogenous substrates. J Biochem. 1988 Jul;104(1):112–117. doi: 10.1093/oxfordjournals.jbchem.a122404. [DOI] [PubMed] [Google Scholar]
- Moriyama A., Nakanishi M., Takenaka O., Sasaki M. Porcine muscle prolyl endopeptidase: limited proteolysis of tryptic peptides from hemoglobin beta-chains at prolyl and alanyl bonds. Biochim Biophys Acta. 1988 Sep 21;956(2):151–155. doi: 10.1016/0167-4838(88)90261-0. [DOI] [PubMed] [Google Scholar]
- Moriyama A., Sasaki M. Porcine liver succinyltrialanine p-nitroanilide hydrolytic enzyme. Its purification and characterization as a post-proline cleaving enzyme. J Biochem. 1983 Nov;94(5):1387–1397. doi: 10.1093/oxfordjournals.jbchem.a134485. [DOI] [PubMed] [Google Scholar]
- Polgar L. pH-dependent mechanism in the catalysis of prolyl endopeptidase from pig muscle. Eur J Biochem. 1991 Apr 23;197(2):441–447. doi: 10.1111/j.1432-1033.1991.tb15930.x. [DOI] [PubMed] [Google Scholar]
- Rennex D., Hemmings B. A., Hofsteenge J., Stone S. R. cDNA cloning of porcine brain prolyl endopeptidase and identification of the active-site seryl residue. Biochemistry. 1991 Feb 26;30(8):2195–2203. doi: 10.1021/bi00222a025. [DOI] [PubMed] [Google Scholar]
- Sattar A. K., Yamamoto N., Yoshimoto T., Tsuru D. Purification and characterization of an extracellular prolyl endopeptidase from Agaricus bisporus. J Biochem. 1990 Feb;107(2):256–261. doi: 10.1093/oxfordjournals.jbchem.a123035. [DOI] [PubMed] [Google Scholar]
- Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967 Apr 20;27(2):157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Schönlein C., Heins J., Barth A. Purification and characterization of prolyl endopeptidase from pig brain. Biol Chem Hoppe Seyler. 1990 Dec;371(12):1159–1164. doi: 10.1515/bchm3.1990.371.2.1159. [DOI] [PubMed] [Google Scholar]
- Soeda S., Ohyama M., Nagamatsu A. A succinyl-trialanine p-nitroanilide hydrolase in hog kidney cytosol: its identification as proline endopeptidase. Chem Pharm Bull (Tokyo) 1984 Apr;32(4):1510–1516. doi: 10.1248/cpb.32.1510. [DOI] [PubMed] [Google Scholar]
- Svendsen I., Breddam K. Isolation and amino acid sequence of a glutamic acid specific endopeptidase from Bacillus licheniformis. Eur J Biochem. 1992 Feb 15;204(1):165–171. doi: 10.1111/j.1432-1033.1992.tb16619.x. [DOI] [PubMed] [Google Scholar]
- Taylor W. L., Dixon J. E. Catabolism of neuropeptides by a brain proline endopeptidase. Biochem Biophys Res Commun. 1980 May 14;94(1):9–15. doi: 10.1016/s0006-291x(80)80179-3. [DOI] [PubMed] [Google Scholar]
- Walter R. Partial purification and characterization of post-proline cleaving enzyme: enzymatic inactivation of neurohypophyseal hormones by kidney preparations of various species. Biochim Biophys Acta. 1976 Jan 23;422(1):138–158. doi: 10.1016/0005-2744(76)90015-2. [DOI] [PubMed] [Google Scholar]
- Walter R., Simmons W. H., Yoshimoto T. Proline specific endo- and exopeptidases. Mol Cell Biochem. 1980 Apr 18;30(2):111–127. doi: 10.1007/BF00227927. [DOI] [PubMed] [Google Scholar]
- Walter R., Yoshimoto T. Postproline cleaving enzyme: kinetic studies of size and stereospecificity of its active site. Biochemistry. 1978 Oct 3;17(20):4139–4144. doi: 10.1021/bi00613a006. [DOI] [PubMed] [Google Scholar]
- Yamakawa N., Soeda S., Shimeno H., Nagamatsu A. Purification and characterization of proline endopeptidase from rat liver. Chem Pharm Bull (Tokyo) 1986 Jan;34(1):256–263. doi: 10.1248/cpb.34.256. [DOI] [PubMed] [Google Scholar]
- Yoshida N., Tsuruyama S., Nagata K., Hirayama K., Noda K., Makisumi S. Purification and characterization of an acidic amino acid specific endopeptidase of Streptomyces griseus obtained from a commercial preparation (Pronase). J Biochem. 1988 Sep;104(3):451–456. doi: 10.1093/oxfordjournals.jbchem.a122488. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T., Fischl M., Orlowski R. C., Walter R. Post-proline cleaving enzyme and post-proline dipeptidyl aminopeptidase. Comparison of two peptidases with high specificity for proline residues. J Biol Chem. 1978 May 25;253(10):3708–3716. [PubMed] [Google Scholar]
- Yoshimoto T., Nishimura T., Kita T., Tsuru D. Post-proline cleaving enzyme (prolyl endopeptidase) from bovine brain. J Biochem. 1983 Oct;94(4):1179–1190. doi: 10.1093/oxfordjournals.jbchem.a134463. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T., Oyama H., Koriyama N., Tsuru D. Prolyl endopeptidase from bovine testis: purification, characterization and comparison with the enzymes from other tissues. Chem Pharm Bull (Tokyo) 1988 Apr;36(4):1456–1462. doi: 10.1248/cpb.36.1456. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T., Sattar A. K., Hirose W., Tsuru D. Studies on prolyl endopeptidase from shakashimeji (Lyophyllum cinerascens): purification and enzymatic properties. J Biochem. 1988 Oct;104(4):622–627. doi: 10.1093/oxfordjournals.jbchem.a122522. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T., Walter R., Tsuru D. Proline-specific endopeptidase from Flavobacterium. Purification and properties. J Biol Chem. 1980 May 25;255(10):4786–4792. [PubMed] [Google Scholar]