Abstract
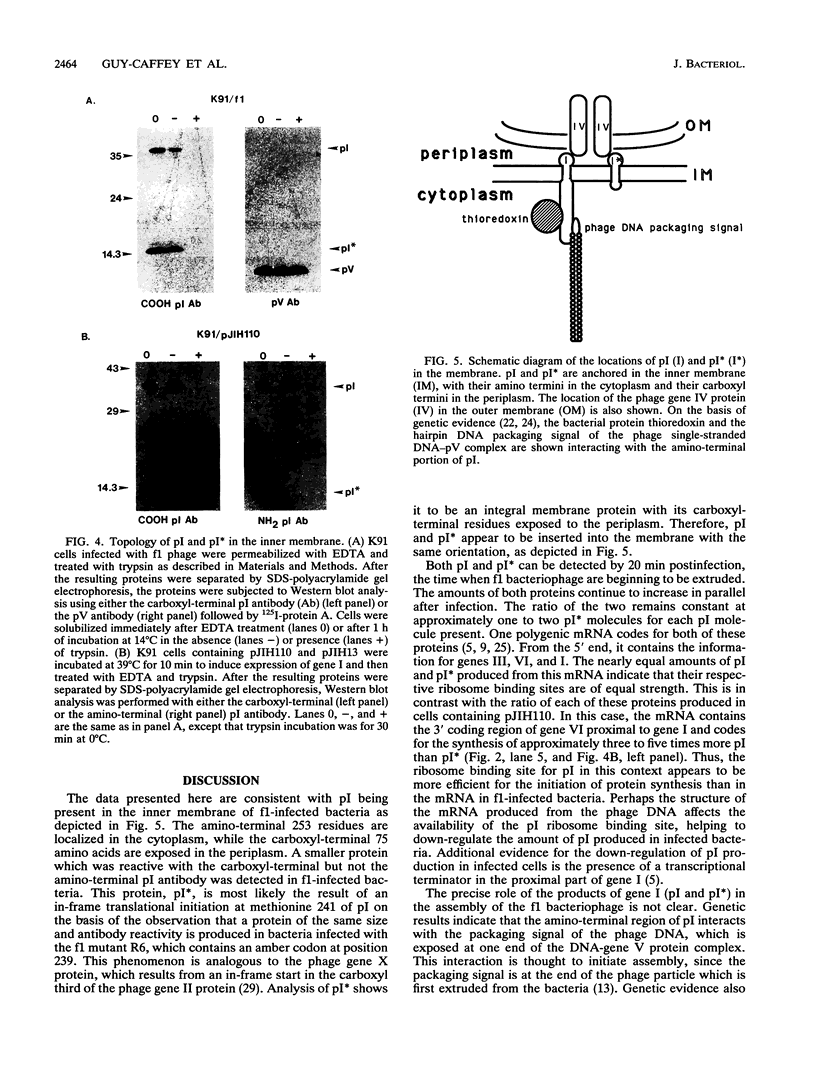
The gene I protein (pI) of the filamentous bacteriophage f1 is required for the assembly of this virus. Antibodies specific to either the amino or carboxyl terminus of this protein were used to determine the location and topology of the gene I protein in f1-infected bacteria. pI is anchored in the inner membrane of Escherichia coli cells via a 20-amino-acid hydrophobic stretch, with its carboxyl-terminal 75 residues located in the periplasm and its amino-terminal 253 amino acids residing in the cytoplasm. By using the carboxyl-terminal pI antibody, a smaller protein, pI*, is also detected in f1-infected cells at a ratio of one to two molecules per molecule of pI. Analysis of proteins produced from a gene I amber mutant plasmid or bacteriophage suggests that pI* is most likely the result of an in-frame internal translational initiation event at methionine 241 of the 348-amino-acid pI. pI* is shown to be an integral inner membrane protein inserted in the same orientation as pI. The relation of the cellular locations of pI and pI* to some of the proposed functions of pI is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer M., Smith G. P. Filamentous phage morphogenetic signal sequence and orientation of DNA in the virion and gene-V protein complex. Virology. 1988 Nov;167(1):166–175. doi: 10.1016/0042-6822(88)90066-9. [DOI] [PubMed] [Google Scholar]
- Bayer M. E. Areas of adhesion between wall and membrane of Escherichia coli. J Gen Microbiol. 1968 Oct;53(3):395–404. doi: 10.1099/00221287-53-3-395. [DOI] [PubMed] [Google Scholar]
- Brissette J. L., Russel M. Secretion and membrane integration of a filamentous phage-encoded morphogenetic protein. J Mol Biol. 1990 Feb 5;211(3):565–580. doi: 10.1016/0022-2836(90)90266-O. [DOI] [PubMed] [Google Scholar]
- Dotto G. P., Enea V., Zinder N. D. Functional analysis of bacteriophage f1 intergenic region. Virology. 1981 Oct 30;114(2):463–473. doi: 10.1016/0042-6822(81)90226-9. [DOI] [PubMed] [Google Scholar]
- Horabin J. I., Webster R. E. An amino acid sequence which directs membrane insertion causes loss of membrane potential. J Biol Chem. 1988 Aug 15;263(23):11575–11583. [PubMed] [Google Scholar]
- Horabin J. I., Webster R. E. Morphogenesis of f1 filamentous bacteriophage. Increased expression of gene I inhibits bacterial growth. J Mol Biol. 1986 Apr 5;188(3):403–413. doi: 10.1016/0022-2836(86)90164-6. [DOI] [PubMed] [Google Scholar]
- Ishidate K., Creeger E. S., Zrike J., Deb S., Glauner B., MacAlister T. J., Rothfield L. I. Isolation of differentiated membrane domains from Escherichia coli and Salmonella typhimurium, including a fraction containing attachment sites between the inner and outer membranes and the murein skeleton of the cell envelope. J Biol Chem. 1986 Jan 5;261(1):428–443. [PubMed] [Google Scholar]
- La Farina M., Model P. Transcription in bacteriophage f1-infected Escherichia coli. Messenger populations in the infected cell. J Mol Biol. 1983 Mar 5;164(3):377–393. doi: 10.1016/0022-2836(83)90057-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levengood S. K., Beyer W. F., Jr, Webster R. E. TolA: a membrane protein involved in colicin uptake contains an extended helical region. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):5939–5943. doi: 10.1073/pnas.88.14.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levengood S. K., Webster R. E. Nucleotide sequences of the tolA and tolB genes and localization of their products, components of a multistep translocation system in Escherichia coli. J Bacteriol. 1989 Dec;171(12):6600–6609. doi: 10.1128/jb.171.12.6600-6609.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C. J., Haller B., Fuchs J. A. Thioredoxin is the bacterial protein encoded by fip that is required for filamentous bacteriophage f1 assembly. J Bacteriol. 1985 Feb;161(2):799–802. doi: 10.1128/jb.161.2.799-802.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J., Webster R. E. Assembly site of bacteriophage f1 corresponds to adhesion zones between the inner and outer membranes of the host cell. J Bacteriol. 1985 Sep;163(3):1270–1274. doi: 10.1128/jb.163.3.1270-1274.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J., Webster R. E. Morphogenesis of filamentous bacteriophage f1: orientation of extrusion and production of polyphage. Virology. 1983 May;127(1):177–193. doi: 10.1016/0042-6822(83)90382-3. [DOI] [PubMed] [Google Scholar]
- Lyons L. B., Zinder N. D. The genetic map of the filamentous bacteriophage f1. Virology. 1972 Jul;49(1):45–60. doi: 10.1016/s0042-6822(72)80006-0. [DOI] [PubMed] [Google Scholar]
- Rasched I., Oberer E. Ff coliphages: structural and functional relationships. Microbiol Rev. 1986 Dec;50(4):401–427. doi: 10.1128/mr.50.4.401-427.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Russel M. Filamentous phage assembly. Mol Microbiol. 1991 Jul;5(7):1607–1613. doi: 10.1111/j.1365-2958.1991.tb01907.x. [DOI] [PubMed] [Google Scholar]
- Russel M., Model P. A bacterial gene, fip, required for filamentous bacteriophage fl assembly. J Bacteriol. 1983 Jun;154(3):1064–1076. doi: 10.1128/jb.154.3.1064-1076.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel M., Model P. Genetic analysis of the filamentous bacteriophage packaging signal and of the proteins that interact with it. J Virol. 1989 Aug;63(8):3284–3295. doi: 10.1128/jvi.63.8.3284-3295.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel M., Model P. The role of thioredoxin in filamentous phage assembly. Construction, isolation, and characterization of mutant thioredoxins. J Biol Chem. 1986 Nov 15;261(32):14997–15005. [PubMed] [Google Scholar]
- Russel M., Model P. Thioredoxin is required for filamentous phage assembly. Proc Natl Acad Sci U S A. 1985 Jan;82(1):29–33. doi: 10.1073/pnas.82.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits M. A., Jansen J., Konings R. N., Schoenmakers J. G. Initiation and termination signals for transcription in bacteriophage M13. Nucleic Acids Res. 1984 May 25;12(10):4071–4081. doi: 10.1093/nar/12.10.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen T. S., Webster R. E. Bacteriophage f1 gene II and X proteins. Isolation and characterization of the products of two overlapping genes. J Biol Chem. 1981 Nov 10;256(21):11259–11265. [PubMed] [Google Scholar]