Abstract
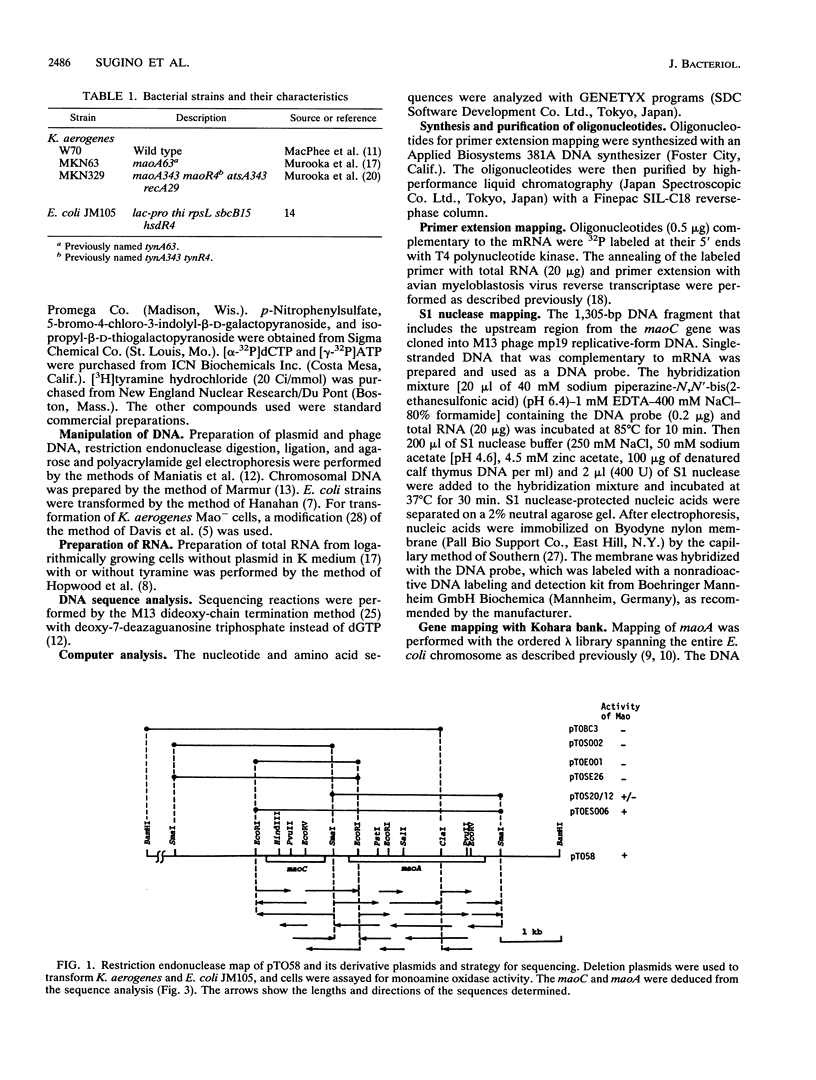
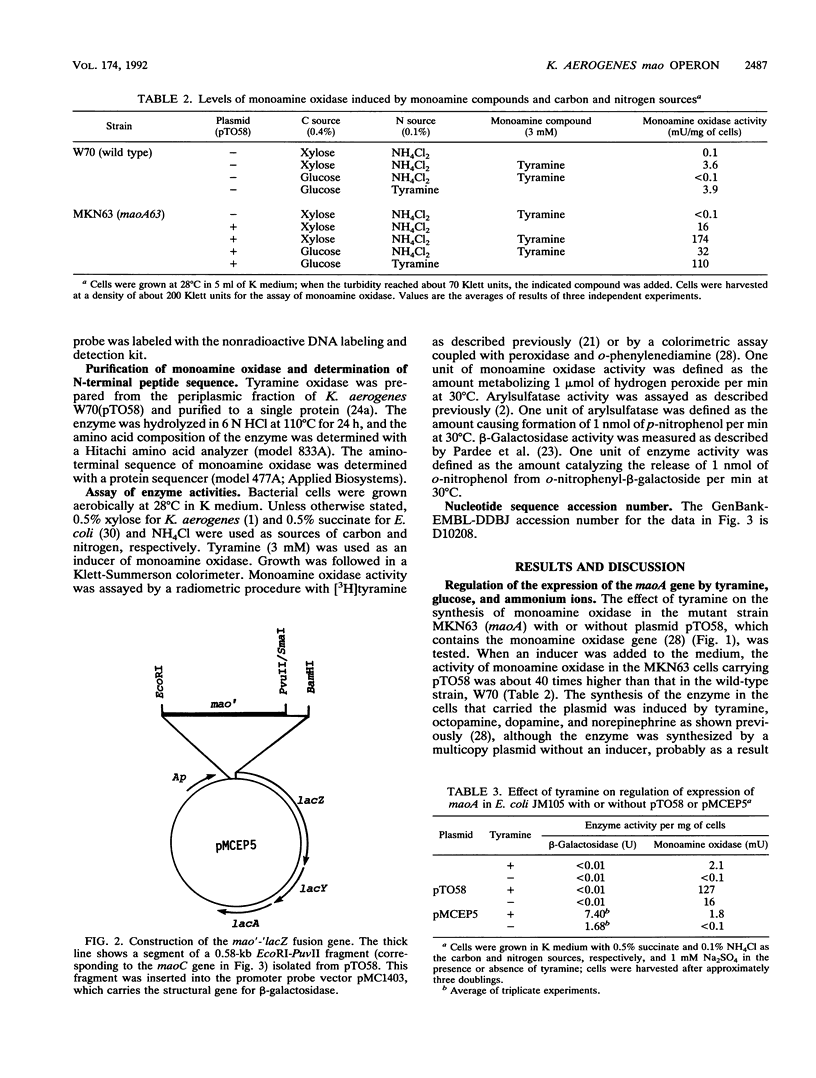
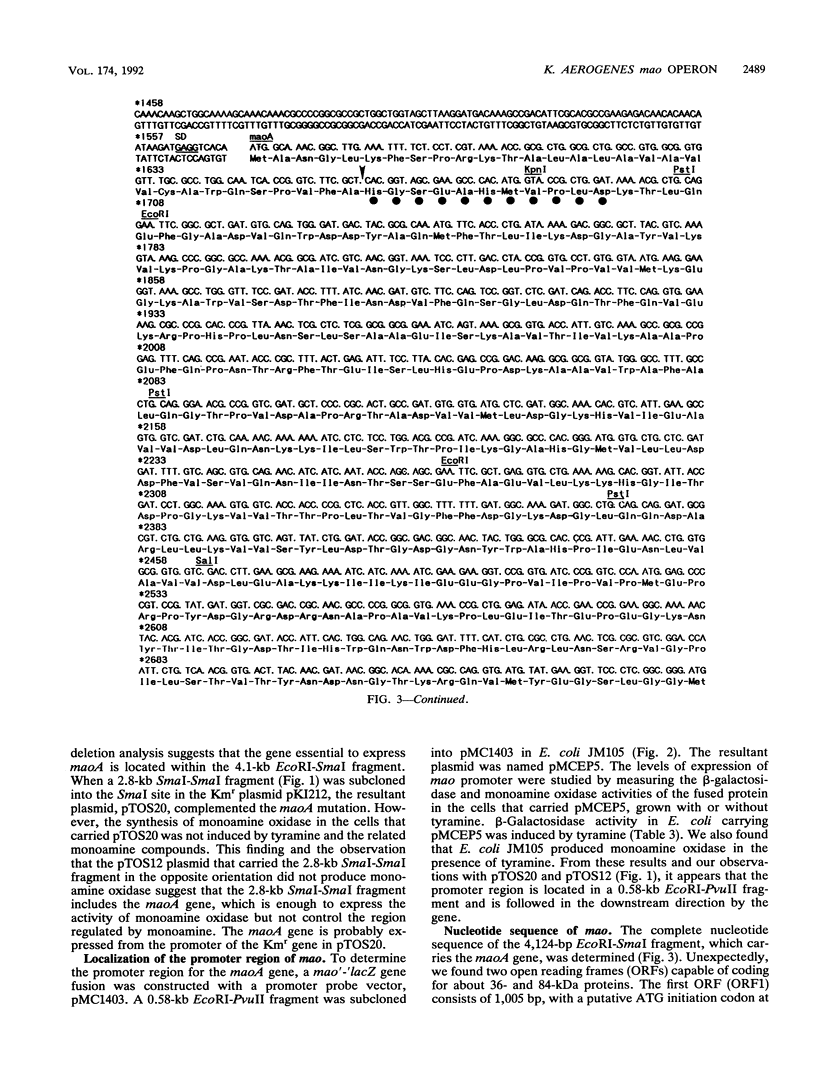
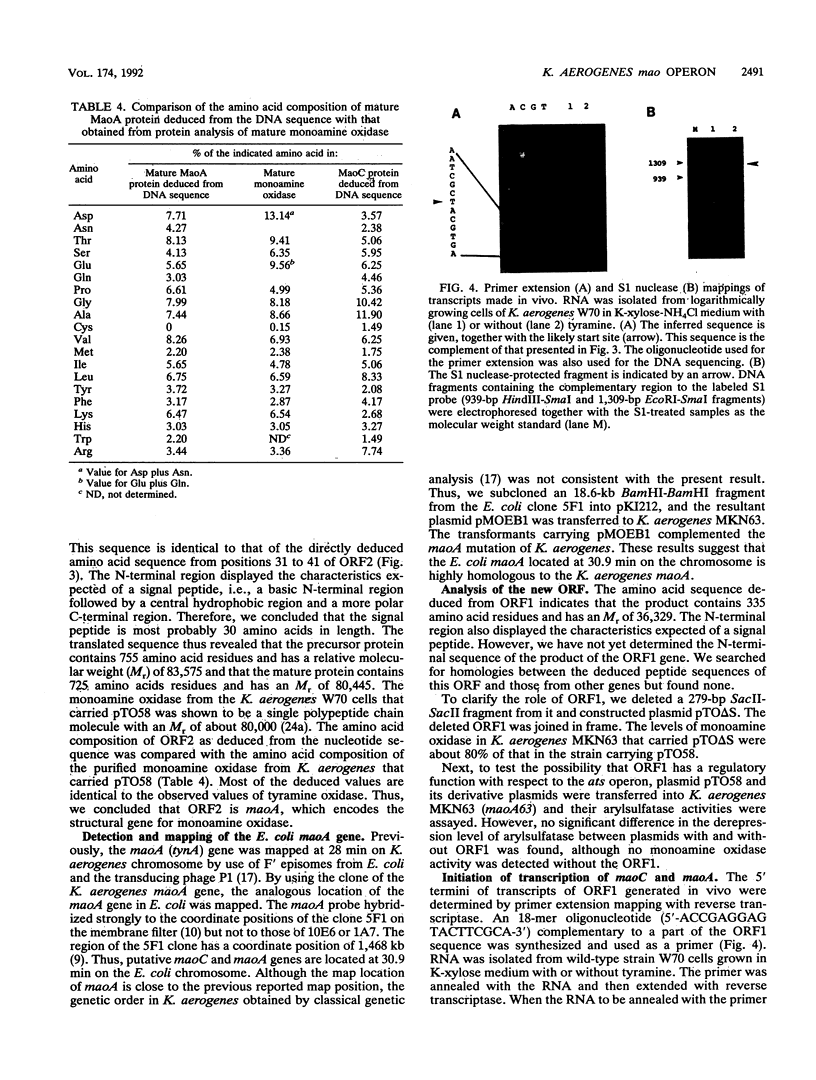
The Klebsiella aerogenes gene maoA, which is involved in the synthesis of monoamine oxidase, was induced by tyramine and the related compounds, subjected to catabolite and ammonium ion repression, and cloned. The nucleotide sequence of the region involved in monoamine oxidase synthesis was determined. Two open reading frames, the maoA gene and a hitherto unknown gene (maoC), were found. These are located between a potential promoter sequence and a transcriptional terminator sequence. A region of the Escherichia coli chromosome that was highly homologous to the Klebsiella maoA gene was found. The potential maoA gene is located at 30.9 min on the E. coli chromosome. Analysis of the amino acid sequences of the first 11 amino acids from the N terminus of the purified monoamine oxidase agrees with those deduced from the nucleotide sequence of the maoA gene. The leader peptide extends over 30 amino acids and has the characteristics of a signal sequence. Primer extension and S1 nuclease mapping of transcripts generated in vivo suggests that the tyramine-induced mRNA starts at a site 62 bases upstream from the ATG initiation codon of the maoC gene. In the putative promoter region, a high degree of similarity to the consensus sequence for the binding site of cyclic AMP receptor protein was found. Thus, the mao region is composed of two cistrons, and the mao operon is regulated by monoamine compounds, glucose, and ammonium ions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi T., Murooka Y., Harada T. Regulation of arylsulfatase synthesis by sulfur compounds in Klebsiella aerogenes. J Bacteriol. 1975 Jan;121(1):29–35. doi: 10.1128/jb.121.1.29-35.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi T., Okamura H., Murooka Y., Harada T. Catabolite repression and derepression of arylsulfatase synthesis in Klebsiella aerogenes. J Bacteriol. 1974 Nov;120(2):880–885. doi: 10.1128/jb.120.2.880-885.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Lee C. C., Kohara Y., Akiyama K., Smith C. L., Craigen W. J., Caskey C. T. Rapid and precise mapping of the Escherichia coli release factor genes by two physical approaches. J Bacteriol. 1988 Oct;170(10):4537–4541. doi: 10.1128/jb.170.10.4537-4541.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPhee D. G., Sutherland I. W., Wilkinson J. F. Transduction in Klebsiella. Nature. 1969 Feb 1;221(5179):475–476. doi: 10.1038/221475a0. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murooka Y., Adachi T., Okamura H., Harada T. Genetic control of arylsulfatase synthesis in Klebsiella aerogenes. J Bacteriol. 1977 Apr;130(1):74–81. doi: 10.1128/jb.130.1.74-81.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murooka Y., Doi N., Harada T. Distribution of membrane-bound monoamine oxidase in bacteria. Appl Environ Microbiol. 1979 Oct;38(4):565–569. doi: 10.1128/aem.38.4.565-569.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murooka Y., Higashiura T., Harada T. Genetic mapping of tyramine oxidase and arylsulfatase genes and their regulation in intergeneric hybrids of enteric bacteria. J Bacteriol. 1978 Nov;136(2):714–722. doi: 10.1128/jb.136.2.714-722.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murooka Y., Ishibashi K., Yasumoto M., Sasaki M., Sugino H., Azakami H., Yamashita M. A sulfur- and tyramine-regulated Klebsiella aerogenes operon containing the arylsulfatase (atsA) gene and the atsB gene. J Bacteriol. 1990 Apr;172(4):2131–2140. doi: 10.1128/jb.172.4.2131-2140.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murooka Y., Yamada T., Tanabe S., Harada T. Immunological study of the regulation of cellular arylsulfatase synthesis in Klebsiella aerogenes. J Bacteriol. 1977 Oct;132(1):247–253. doi: 10.1128/jb.132.1.247-253.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka M., Murooka Y., Harada T. Genetic control of tyramine oxidase, which is involved in derepressed synthesis of arylsulfatase in Klebsiella aerogenes. J Bacteriol. 1980 Jul;143(1):321–327. doi: 10.1128/jb.143.1.321-327.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura H., Murooka Y., Harada T. Regulation of tyramine oxidase synthesis in Klebsiella aerogenes. J Bacteriol. 1976 Jul;127(1):24–31. doi: 10.1128/jb.127.1.24-31.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura H., Murooka Y., Harada T. Tyramine oxidase and regulation of arylsulfatase synthesis in Klebsiella aerogenes. J Bacteriol. 1977 Jan;129(1):59–65. doi: 10.1128/jb.129.1.59-65.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sugino H., Ishibashi K., Sakaue M., Yamashita M., Murooka Y. Gene cloning of the maoA gene and overproduction of a soluble monoamine oxidase from Klebsiella aerogenes. Appl Microbiol Biotechnol. 1991 Aug;35(5):606–610. doi: 10.1007/BF00169624. [DOI] [PubMed] [Google Scholar]
- de Crombrugghe B., Busby S., Buc H. Cyclic AMP receptor protein: role in transcription activation. Science. 1984 May 25;224(4651):831–838. doi: 10.1126/science.6372090. [DOI] [PubMed] [Google Scholar]




