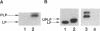Abstract
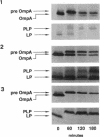
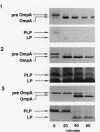
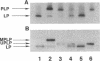
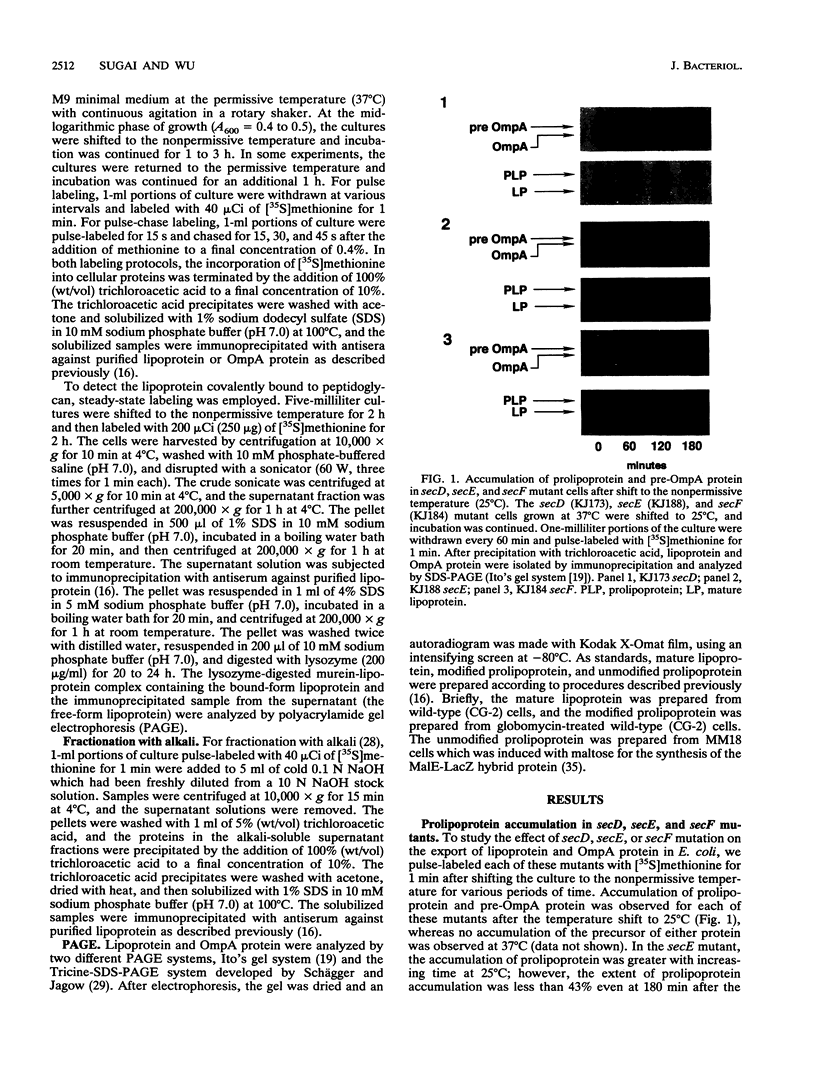
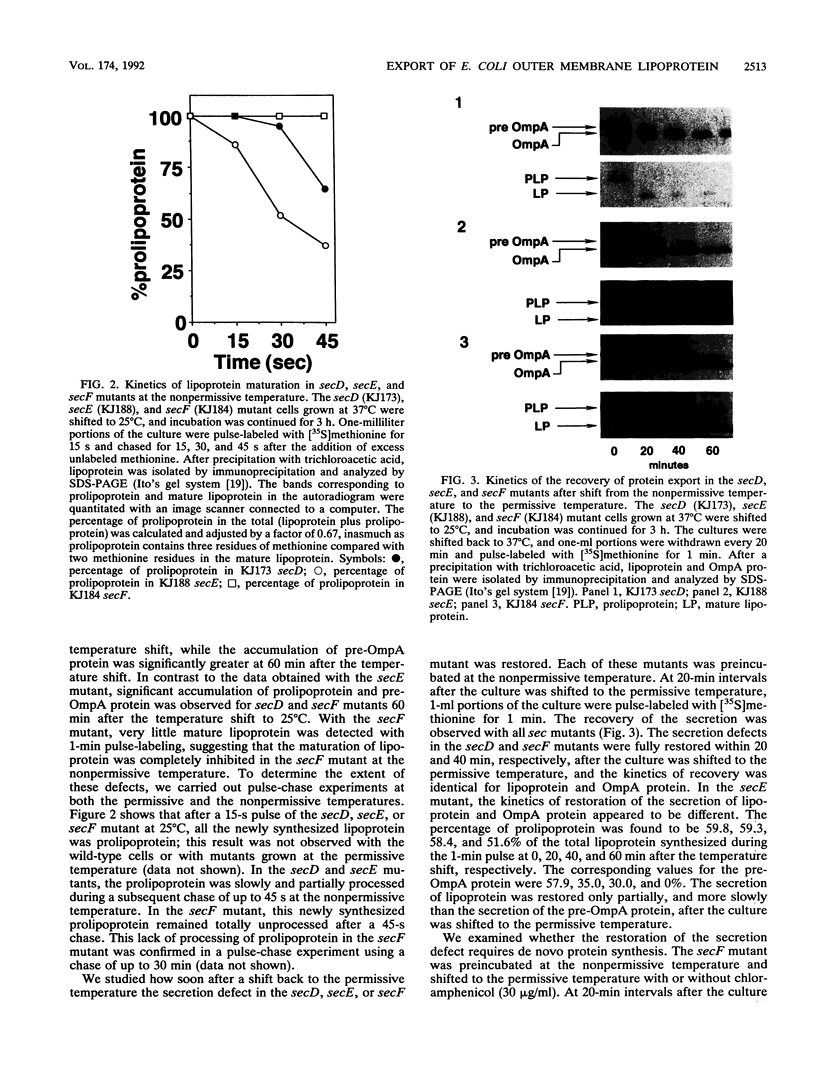
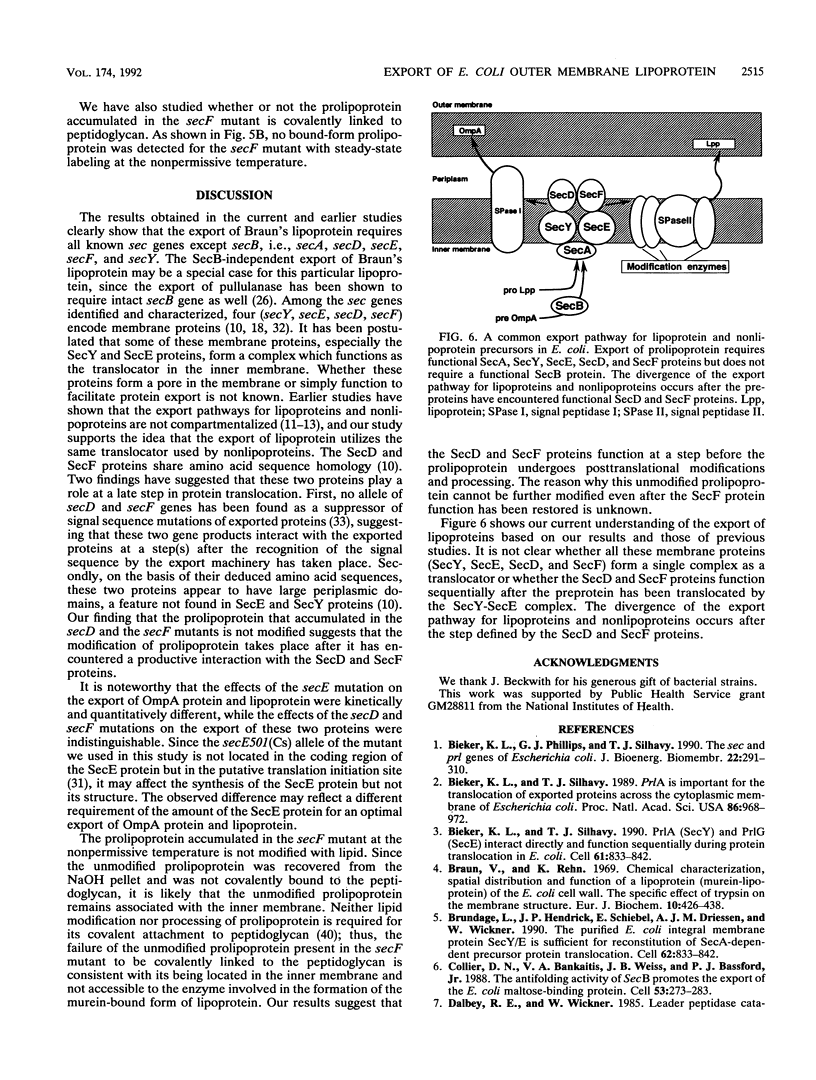
The export of major outer membrane lipoprotein has been found to be affected in secD, secE, and secF mutants of Escherichia coli, which are defective in protein export in general. After a shift to the nonpermissive temperature, the kinetics of accumulation of prolipoprotein and pre-OmpA protein was indistinguishable from that of pre-OmpA protein accumulation in the secD and secF mutants but different in the secE mutant. The prolipoprotein accumulated in the secD, secE, and secF mutants at the nonpermissive temperature was not modified with glyceride. We conclude from these results and those of previous studies that the export of lipoprotein requires all common sec gene products except the SecB protein, i.e., the SecA, SecD, SecE, SecF, and SecY proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bieker K. L., Phillips G. J., Silhavy T. J. The sec and prl genes of Escherichia coli. J Bioenerg Biomembr. 1990 Jun;22(3):291–310. doi: 10.1007/BF00763169. [DOI] [PubMed] [Google Scholar]
- Bieker K. L., Silhavy T. J. PrlA (SecY) and PrlG (SecE) interact directly and function sequentially during protein translocation in E. coli. Cell. 1990 Jun 1;61(5):833–842. doi: 10.1016/0092-8674(90)90193-i. [DOI] [PubMed] [Google Scholar]
- Bieker K. L., Silhavy T. J. PrlA is important for the translocation of exported proteins across the cytoplasmic membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1989 Feb;86(3):968–972. doi: 10.1073/pnas.86.3.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Collier D. N., Bankaitis V. A., Weiss J. B., Bassford P. J., Jr The antifolding activity of SecB promotes the export of the E. coli maltose-binding protein. Cell. 1988 Apr 22;53(2):273–283. doi: 10.1016/0092-8674(88)90389-3. [DOI] [PubMed] [Google Scholar]
- Dalbey R. E., Wickner W. Leader peptidase catalyzes the release of exported proteins from the outer surface of the Escherichia coli plasma membrane. J Biol Chem. 1985 Dec 15;260(29):15925–15931. [PubMed] [Google Scholar]
- Dev I. K., Ray P. H. Rapid assay and purification of a unique signal peptidase that processes the prolipoprotein from Escherichia coli B. J Biol Chem. 1984 Sep 10;259(17):11114–11120. [PubMed] [Google Scholar]
- Gardel C., Benson S., Hunt J., Michaelis S., Beckwith J. secD, a new gene involved in protein export in Escherichia coli. J Bacteriol. 1987 Mar;169(3):1286–1290. doi: 10.1128/jb.169.3.1286-1290.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardel C., Johnson K., Jacq A., Beckwith J. The secD locus of E.coli codes for two membrane proteins required for protein export. EMBO J. 1990 Oct;9(10):3209–3216. doi: 10.1002/j.1460-2075.1990.tb07519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghrayeb J., Lunn C. A., Inouye S., Inouye M. An alternate pathway for the processing of the prolipoprotein signal peptide in Escherichia coli. J Biol Chem. 1985 Sep 15;260(20):10961–10965. [PubMed] [Google Scholar]
- Hayashi S., Chang S. Y., Chang S., Wu H. C. Modification and processing of Bacillus licheniformis prepenicillinase in Escherichia coli. Fate of mutant penicillinase lacking lipoprotein modification site. J Biol Chem. 1984 Aug 25;259(16):10448–10454. [PubMed] [Google Scholar]
- Hayashi S., Chang S. Y., Chang S., Wu H. C. Processing of Bacillus licheniformis penicillinases lacking a lipoprotein modification site in Escherichia coli. J Bacteriol. 1986 Mar;165(3):678–681. doi: 10.1128/jb.165.3.678-681.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S., Wu H. C. Accumulation of prolipoprotein in Escherichia coli mutants defective in protein secretion. J Bacteriol. 1985 Mar;161(3):949–954. doi: 10.1128/jb.161.3.949-954.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S., Wu H. C. Lipoproteins in bacteria. J Bioenerg Biomembr. 1990 Jun;22(3):451–471. doi: 10.1007/BF00763177. [DOI] [PubMed] [Google Scholar]
- Hussain M., Ichihara S., Mizushima S. Mechanism of signal peptide cleavage in the biosynthesis of the major lipoprotein of the Escherichia coli outer membrane. J Biol Chem. 1982 May 10;257(9):5177–5182. [PubMed] [Google Scholar]
- Ito K., Date T., Wickner W. Synthesis, assembly into the cytoplasmic membrane, and proteolytic processing of the precursor of coliphage M13 coat protein. J Biol Chem. 1980 Mar 10;255(5):2123–2130. [PubMed] [Google Scholar]
- Ito K., Wittekind M., Nomura M., Shiba K., Yura T., Miura A., Nashimoto H. A temperature-sensitive mutant of E. coli exhibiting slow processing of exported proteins. Cell. 1983 Mar;32(3):789–797. doi: 10.1016/0092-8674(83)90065-x. [DOI] [PubMed] [Google Scholar]
- Kumamoto C. A. Molecular chaperones and protein translocation across the Escherichia coli inner membrane. Mol Microbiol. 1991 Jan;5(1):19–22. doi: 10.1111/j.1365-2958.1991.tb01821.x. [DOI] [PubMed] [Google Scholar]
- Kusukawa N., Yura T., Ueguchi C., Akiyama Y., Ito K. Effects of mutations in heat-shock genes groES and groEL on protein export in Escherichia coli. EMBO J. 1989 Nov;8(11):3517–3521. doi: 10.1002/j.1460-2075.1989.tb08517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecker S., Lill R., Ziegelhoffer T., Georgopoulos C., Bassford P. J., Jr, Kumamoto C. A., Wickner W. Three pure chaperone proteins of Escherichia coli--SecB, trigger factor and GroEL--form soluble complexes with precursor proteins in vitro. EMBO J. 1989 Sep;8(9):2703–2709. doi: 10.1002/j.1460-2075.1989.tb08411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. E. coli mutant pleiotropically defective in the export of secreted proteins. Cell. 1981 Sep;25(3):765–772. doi: 10.1016/0092-8674(81)90184-7. [DOI] [PubMed] [Google Scholar]
- Phillips G. J., Silhavy T. J. Heat-shock proteins DnaK and GroEL facilitate export of LacZ hybrid proteins in E. coli. Nature. 1990 Apr 26;344(6269):882–884. doi: 10.1038/344882a0. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Kornacker M. G., Poquet I. The general protein-export pathway is directly required for extracellular pullulanase secretion in Escherichia coli K12. Mol Microbiol. 1991 Feb;5(2):343–352. doi: 10.1111/j.1365-2958.1991.tb02115.x. [DOI] [PubMed] [Google Scholar]
- Riggs P. D., Derman A. I., Beckwith J. A mutation affecting the regulation of a secA-lacZ fusion defines a new sec gene. Genetics. 1988 Apr;118(4):571–579. doi: 10.1093/genetics/118.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel M., Model P. Filamentous phage pre-coat is an integral membrane protein: analysis by a new method of membrane preparation. Cell. 1982 Jan;28(1):177–184. doi: 10.1016/0092-8674(82)90387-7. [DOI] [PubMed] [Google Scholar]
- Schatz P. J., Beckwith J. Genetic analysis of protein export in Escherichia coli. Annu Rev Genet. 1990;24:215–248. doi: 10.1146/annurev.ge.24.120190.001243. [DOI] [PubMed] [Google Scholar]
- Schatz P. J., Bieker K. L., Ottemann K. M., Silhavy T. J., Beckwith J. One of three transmembrane stretches is sufficient for the functioning of the SecE protein, a membrane component of the E. coli secretion machinery. EMBO J. 1991 Jul;10(7):1749–1757. doi: 10.1002/j.1460-2075.1991.tb07699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz P. J., Riggs P. D., Jacq A., Fath M. J., Beckwith J. The secE gene encodes an integral membrane protein required for protein export in Escherichia coli. Genes Dev. 1989 Jul;3(7):1035–1044. doi: 10.1101/gad.3.7.1035. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Stader J., Gansheroff L. J., Silhavy T. J. New suppressors of signal-sequence mutations, prlG, are linked tightly to the secE gene of Escherichia coli. Genes Dev. 1989 Jul;3(7):1045–1052. doi: 10.1101/gad.3.7.1045. [DOI] [PubMed] [Google Scholar]
- Tokunaga M., Loranger J. M., Wolfe P. B., Wu H. C. Prolipoprotein signal peptidase in Escherichia coli is distinct from the M13 procoat protein signal peptidase. J Biol Chem. 1982 Sep 10;257(17):9922–9925. [PubMed] [Google Scholar]
- Tokunaga M., Tokunaga H., Wu H. C. Post-translational modification and processing of Escherichia coli prolipoprotein in vitro. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2255–2259. doi: 10.1073/pnas.79.7.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Blobel G. SecB functions as a cytosolic signal recognition factor for protein export in E. coli. Cell. 1989 Aug 25;58(4):695–705. doi: 10.1016/0092-8674(89)90104-9. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Hayashi S., Wu H. C. Synthesis and export of the outer membrane lipoprotein in Escherichia coli mutants defective in generalized protein export. J Bacteriol. 1988 Sep;170(9):4001–4007. doi: 10.1128/jb.170.9.4001-4007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe P. B., Silver P., Wickner W. The isolation of homogeneous leader peptidase from a strain of Escherichia coli which overproduces the enzyme. J Biol Chem. 1982 Jul 10;257(13):7898–7902. [PubMed] [Google Scholar]
- Wolfe P. B., Wickner W., Goodman J. M. Sequence of the leader peptidase gene of Escherichia coli and the orientation of leader peptidase in the bacterial envelope. J Biol Chem. 1983 Oct 10;258(19):12073–12080. [PubMed] [Google Scholar]