Abstract
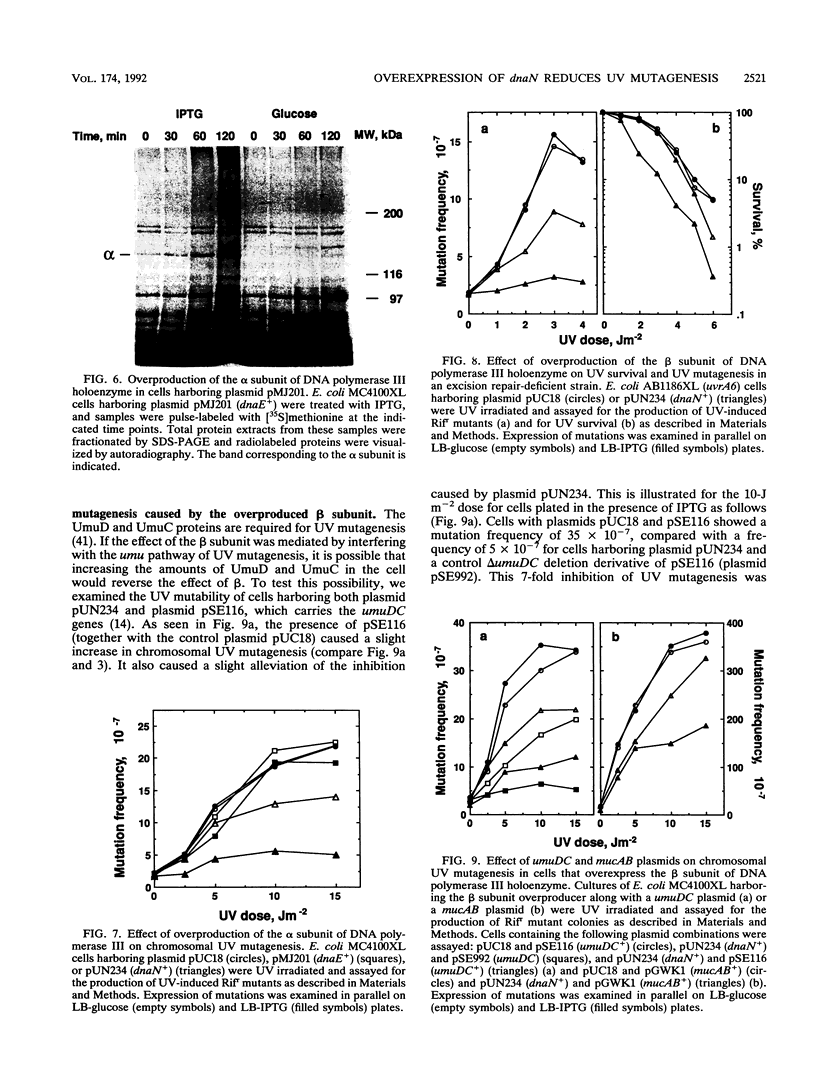
Overproduction of the beta subunit of DNA polymerase III holoenzyme caused a 5- to 10-fold reduction of UV mutagenesis along with a slight increase in sensitivity to UV light in Escherichia coli. The same effects were observed in excision-deficient cells, excluding the possibility that they were mediated via changes in excision repair. In contrast, overproduction of the alpha subunit of the polymerase did not influence either UV mutagenesis or UV sensitivity. The presence of the mutagenesis proteins MucA and MucB expressed from a plasmid alleviated the effect of overproduced beta on UV mutagenesis. We have previously suggested that DNA polymerase III holoenzyme can exist in two forms: beta-rich form unable to bypass UV lesions and a beta-poor form capable of bypassing UV lesions (O. Shavitt and Z. Livneh, J. Biol. Chem. 264:11275-11281, 1989). The beta-poor form may be related to an SOS form of DNA polymerase III designed to perform translesion polymerization under SOS conditions and thereby generate mutations. On the basis of this model, we propose that the overproduced beta subunit affects the relative abundance of the regular replicative beta-rich polymerase and the SOS bypass-proficient polymerase by sequestering the polymerase molecules to the beta-rich form and blocking the SOS form.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee S. K., Christensen R. B., Lawrence C. W., LeClerc J. E. Frequency and spectrum of mutations produced by a single cis-syn thymine-thymine cyclobutane dimer in a single-stranded vector. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8141–8145. doi: 10.1073/pnas.85.21.8141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates H., Randall S. K., Rayssiguier C., Bridges B. A., Goodman M. F., Radman M. Spontaneous and UV-induced mutations in Escherichia coli K-12 strains with altered or absent DNA polymerase I. J Bacteriol. 1989 May;171(5):2480–2484. doi: 10.1128/jb.171.5.2480-2484.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanar M. A., Sandler S. J., Armengod M. E., Ream L. W., Clark A. J. Molecular analysis of the recF gene of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4622–4626. doi: 10.1073/pnas.81.15.4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco M., Herrera G., Aleixandre V. Different efficiency of UmuDC and MucAB proteins in UV light induced mutagenesis in Escherichia coli. Mol Gen Genet. 1986 Nov;205(2):234–239. doi: 10.1007/BF00430433. [DOI] [PubMed] [Google Scholar]
- Bonner C. A., Hays S., McEntee K., Goodman M. F. DNA polymerase II is encoded by the DNA damage-inducible dinA gene of Escherichia coli. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7663–7667. doi: 10.1073/pnas.87.19.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges B. A., Bates H. Mutagenic DNA repair in Escherichia coli. XVIII. Involvement of DNA polymerase III alpha-subunit (DnaE protein) in mutagenesis after exposure to UV light. Mutagenesis. 1990 Jan;5(1):35–38. doi: 10.1093/mutage/5.1.35. [DOI] [PubMed] [Google Scholar]
- Bridges B. A., Mottershead R. P. Mutagenic DNA repair in Escherichia coli. III. Requirement for a function of DNA polymerase III in ultraviolet-light mutagenesis. Mol Gen Genet. 1976 Feb 27;144(1):53–58. doi: 10.1007/BF00277304. [DOI] [PubMed] [Google Scholar]
- Brotcorne-Lannoye A., Maenhaut-Michel G., Radman M. Involvement of DNA polymerase III in UV-induced mutagenesis of bacteriophage lambda. Mol Gen Genet. 1985;199(1):64–69. doi: 10.1007/BF00327511. [DOI] [PubMed] [Google Scholar]
- Bryan S. K., Hagensee M., Moses R. E. Holoenzyme DNA polymerase III fixes mutations. Mutat Res. 1990 Apr;243(4):313–318. doi: 10.1016/0165-7992(90)90149-e. [DOI] [PubMed] [Google Scholar]
- Burgers P. M., Kornberg A., Sakakibara Y. The dnaN gene codes for the beta subunit of DNA polymerase III holoenzyme of escherichia coli. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5391–5395. doi: 10.1073/pnas.78.9.5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crute J. J., LaDuca R. J., Johanson K. O., McHenry C. S., Bambara R. A. Excess beta subunit can bypass the ATP requirement for highly processive synthesis by the Escherichia coli DNA polymerase III holoenzyme. J Biol Chem. 1983 Sep 25;258(18):11344–11349. [PubMed] [Google Scholar]
- Echols H., Goodman M. F. Mutation induced by DNA damage: a many protein affair. Mutat Res. 1990 Sep-Nov;236(2-3):301–311. doi: 10.1016/0921-8777(90)90013-u. [DOI] [PubMed] [Google Scholar]
- Elledge S. J., Walker G. C. Proteins required for ultraviolet light and chemical mutagenesis. Identification of the products of the umuC locus of Escherichia coli. J Mol Biol. 1983 Feb 25;164(2):175–192. doi: 10.1016/0022-2836(83)90074-8. [DOI] [PubMed] [Google Scholar]
- Hagensee M. E., Timme T. L., Bryan S. K., Moses R. E. DNA polymerase III of Escherichia coli is required for UV and ethyl methanesulfonate mutagenesis. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4195–4199. doi: 10.1073/pnas.84.12.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevroni D., Livneh Z. Bypass and termination at apurinic sites during replication of single-stranded DNA in vitro: a model for apurinic site mutagenesis. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5046–5050. doi: 10.1073/pnas.85.14.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonczyk P., Fijalkowska I., Ciesla Z. Overproduction of the epsilon subunit of DNA polymerase III counteracts the SOS mutagenic response of Escherichia coli. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9124–9127. doi: 10.1073/pnas.85.23.9124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T., Shinoura Y. Isolation and characterization of mutants of Escherichia coli deficient in induction of mutations by ultraviolet light. Mol Gen Genet. 1977 Nov 14;156(2):121–131. doi: 10.1007/BF00283484. [DOI] [PubMed] [Google Scholar]
- Kenyon C. J., Walker G. C. DNA-damaging agents stimulate gene expression at specific loci in Escherichia coli. Proc Natl Acad Sci U S A. 1980 May;77(5):2819–2823. doi: 10.1073/pnas.77.5.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasken R. S., Kornberg A. The beta subunit dissociates readily from the Escherichia coli DNA polymerase III holoenzyme. J Biol Chem. 1987 Feb 5;262(4):1720–1724. [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Livneh Z. Mechanism of replication of ultraviolet-irradiated single-stranded DNA by DNA polymerase III holoenzyme of Escherichia coli. Implications for SOS mutagenesis. J Biol Chem. 1986 Jul 15;261(20):9526–9533. [PubMed] [Google Scholar]
- Maki H., Kornberg A. The polymerase subunit of DNA polymerase III of Escherichia coli. II. Purification of the alpha subunit, devoid of nuclease activities. J Biol Chem. 1985 Oct 25;260(24):12987–12992. [PubMed] [Google Scholar]
- Maki H., Maki S., Kornberg A. DNA Polymerase III holoenzyme of Escherichia coli. IV. The holoenzyme is an asymmetric dimer with twin active sites. J Biol Chem. 1988 May 15;263(14):6570–6578. [PubMed] [Google Scholar]
- Maki S., Kornberg A. DNA polymerase III holoenzyme of Escherichia coli. III. Distinctive processive polymerases reconstituted from purified subunits. J Biol Chem. 1988 May 15;263(14):6561–6569. [PubMed] [Google Scholar]
- McHenry C. S. DNA polymerase III holoenzyme of Escherichia coli. Annu Rev Biochem. 1988;57:519–550. doi: 10.1146/annurev.bi.57.070188.002511. [DOI] [PubMed] [Google Scholar]
- O'Donnell M. E. Accessory proteins bind a primed template and mediate rapid cycling of DNA polymerase III holoenzyme from Escherichia coli. J Biol Chem. 1987 Dec 5;262(34):16558–16565. [PubMed] [Google Scholar]
- O'Donnell M., Studwell P. S. Total reconstitution of DNA polymerase III holoenzyme reveals dual accessory protein clamps. J Biol Chem. 1990 Jan 15;265(2):1179–1187. [PubMed] [Google Scholar]
- Ohmori H., Kimura M., Nagata T., Sakakibara Y. Structural analysis of the dnaA and dnaN genes of Escherichia coli. Gene. 1984 May;28(2):159–170. doi: 10.1016/0378-1119(84)90253-1. [DOI] [PubMed] [Google Scholar]
- Perry K. L., Walker G. C. Identification of plasmid (pKM101)-coded proteins involved in mutagenesis and UV resistance. Nature. 1982 Nov 18;300(5889):278–281. doi: 10.1038/300278a0. [DOI] [PubMed] [Google Scholar]
- Sakakibara Y., Mizukami T. A temperature-sensitive Escherichia coli mutant defective in DNA replication: dnaN, a new gene adjacent to the dnaA gene. Mol Gen Genet. 1980;178(3):541–553. doi: 10.1007/BF00337859. [DOI] [PubMed] [Google Scholar]
- Sedgwick S. G., Goodwin P. A. Differences in mutagenic and recombinational DNA repair in enterobacteria. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4172–4176. doi: 10.1073/pnas.82.12.4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shavitt O., Livneh Z. The beta subunit modulates bypass and termination at UV lesions during in vitro replication with DNA polymerase III holoenzyme of Escherichia coli. J Biol Chem. 1989 Jul 5;264(19):11275–11281. [PubMed] [Google Scholar]
- Shwartz H., Livneh Z. Dynamics of termination during in vitro replication of ultraviolet-irradiated DNA with DNA polymerase III holoenzyme of Escherichia coli. J Biol Chem. 1987 Aug 5;262(22):10518–10523. [PubMed] [Google Scholar]
- Shwartz H., Shavitt O., Livneh Z. The role of exonucleolytic processing and polymerase-DNA association in bypass of lesions during replication in vitro. Significance for SOS-targeted mutagenesis. J Biol Chem. 1988 Dec 5;263(34):18277–18285. [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]