Abstract
Thioredoxin is a small redox protein that functions as a reducing agent and modulator of enzyme activity. A gene for an unusual thioredoxin was previously isolated from the cyanobacterium Anabaena sp. strain PCC 7120 and cloned and expressed in Escherichia coli. However, the protein could not be detected in Anabaena cells (J. Alam, S. Curtis, F. K. Gleason, M. Gerami-Nejad, and J. A. Fuchs, J. Bacteriol. 171:162-171, 1989). Polyclonal antibodies to the atypical thioredoxin were prepared, and the protein was detected by Western immunoblotting. It occurs at very low levels in extracts of Anabaena sp. and other cyanobacteria. No antibody cross-reaction was observed in extracts of eukaryotic algae, plants, or eubacteria. The anti-Anabaena thioredoxin antibodies did react with another unusual thioredoxin-glutaredoxin produced by bacteriophage T4. Like the T4 protein and other glutaredoxins, the unusual cyanobacterial thioredoxin can be reduced by glutathione. The Anabaena protein can also activate enzymes of carbon metabolism and has some functional similarity to spinach chloroplast thioredoxin f. However, it shows only 23% amino acid sequence identity to the spinach chloroplast protein and appears to be distantly related to other thioredoxins. The data indicate that cyanobacteria, like plant chloroplasts, have two dissimilar thioredoxins. One is related to the more common protein found in other prokaryotes, and the other is an unusual thioredoxin that can be reduced by glutathione and may function in glucose catabolism.
Full text
PDF


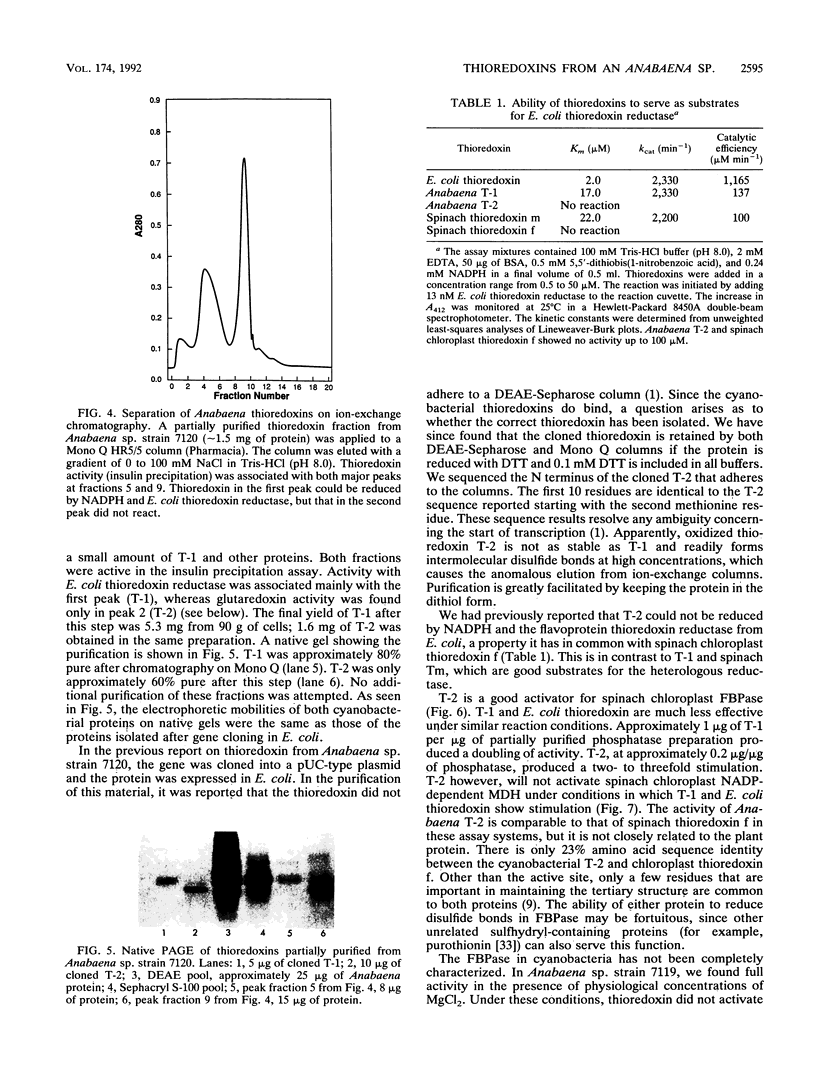
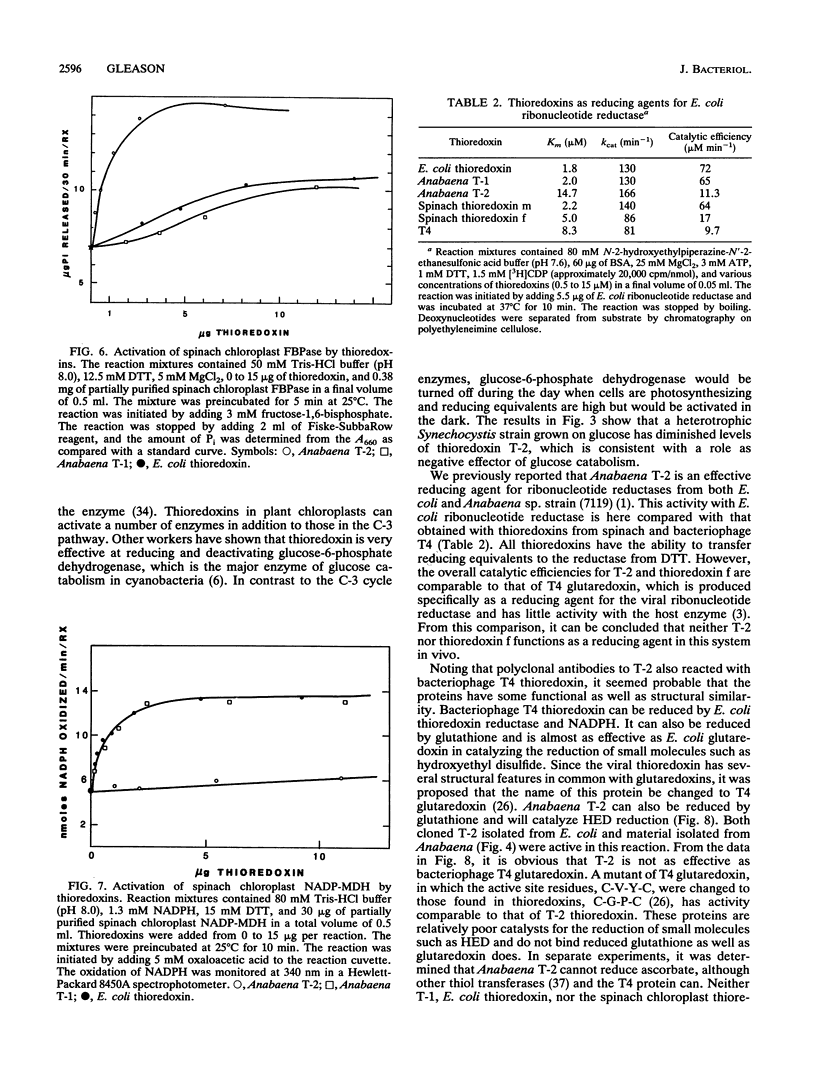
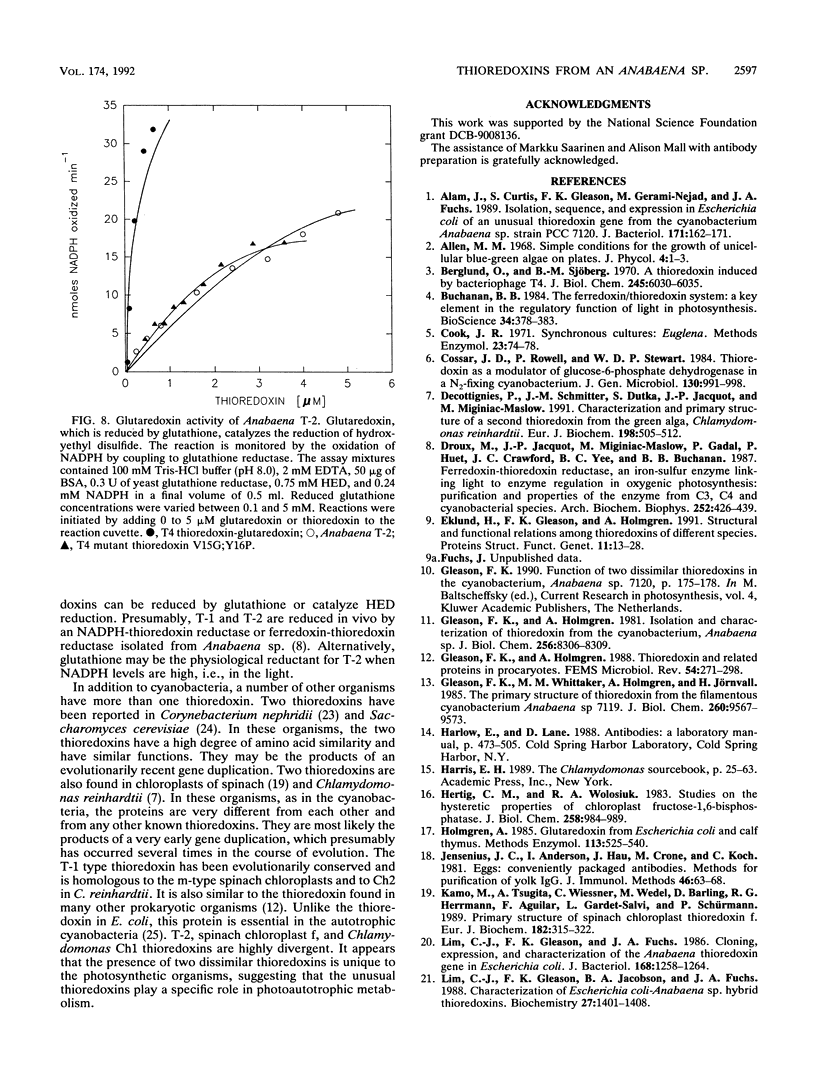

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alam J., Curtis S., Gleason F. K., Gerami-Nejad M., Fuchs J. A. Isolation, sequence, and expression in Escherichia coli of an unusual thioredoxin gene from the cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 1989 Jan;171(1):162–171. doi: 10.1128/jb.171.1.162-171.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund O., Sjöberg B. M. A thioredoxin induced by bacteriophage T4. II. Purification and characterization. J Biol Chem. 1970 Nov 25;245(22):6030–6035. [PubMed] [Google Scholar]
- Buchanan B. B. The ferredoxin/thioredoxin system: a key element in the regulatory function of light in photosynthesis. Bioscience. 1984 Jun;34(6):378–383. [PubMed] [Google Scholar]
- Decottignies P., Schmitter J. M., Dutka S., Jacquot J. P., Miginiac-Maslow M. Characterization and primary structure of a second thioredoxin from the green alga, Chlamydomonas reinhardtii. Eur J Biochem. 1991 Jun 1;198(2):505–512. doi: 10.1111/j.1432-1033.1991.tb16043.x. [DOI] [PubMed] [Google Scholar]
- Droux M., Jacquot J. P., Miginac-Maslow M., Gadal P., Huet J. C., Crawford N. A., Yee B. C., Buchanan B. B. Ferredoxin-thioredoxin reductase, an iron-sulfur enzyme linking light to enzyme regulation in oxygenic photosynthesis: purification and properties of the enzyme from C3, C4, and cyanobacterial species. Arch Biochem Biophys. 1987 Feb 1;252(2):426–439. doi: 10.1016/0003-9861(87)90049-x. [DOI] [PubMed] [Google Scholar]
- Eklund H., Gleason F. K., Holmgren A. Structural and functional relations among thioredoxins of different species. Proteins. 1991;11(1):13–28. doi: 10.1002/prot.340110103. [DOI] [PubMed] [Google Scholar]
- Gleason F. K., Holmgren A. Isolation and characterization of thioredoxin from the cyanobacterium, Anabaena sp. J Biol Chem. 1981 Aug 25;256(16):8306–8309. [PubMed] [Google Scholar]
- Gleason F. K., Holmgren A. Thioredoxin and related proteins in procaryotes. FEMS Microbiol Rev. 1988 Dec;4(4):271–297. doi: 10.1111/j.1574-6968.1988.tb02747.x. [DOI] [PubMed] [Google Scholar]
- Gleason F. K., Whittaker M. M., Holmgren A., Jörnvall H. The primary structure of thioredoxin from the filamentous cyanobacterium Anabaena sp. 7119. J Biol Chem. 1985 Aug 15;260(17):9567–9573. [PubMed] [Google Scholar]
- Hertig C. M., Wolosiuk R. A. Studies on the hysteretic properties of chloroplast fructose-1,6-bisphosphatase. J Biol Chem. 1983 Jan 25;258(2):984–989. [PubMed] [Google Scholar]
- Holmgren A. Glutaredoxin from Escherichia coli and calf thymus. Methods Enzymol. 1985;113:525–540. doi: 10.1016/s0076-6879(85)13071-5. [DOI] [PubMed] [Google Scholar]
- Jensenius J. C., Andersen I., Hau J., Crone M., Koch C. Eggs: conveniently packaged antibodies. Methods for purification of yolk IgG. J Immunol Methods. 1981;46(1):63–68. doi: 10.1016/0022-1759(81)90333-1. [DOI] [PubMed] [Google Scholar]
- Kamo M., Tsugita A., Wiessner C., Wedel N., Bartling D., Herrmann R. G., Aguilar F., Gardet-Salvi L., Schürmann P. Primary structure of spinach-chloroplast thioredoxin f. Protein sequencing and analysis of complete cDNA clones for spinach-chloroplast thioredoxin f. Eur J Biochem. 1989 Jun 15;182(2):315–322. doi: 10.1111/j.1432-1033.1989.tb14832.x. [DOI] [PubMed] [Google Scholar]
- Lim C. J., Gleason F. K., Fuchs J. A. Cloning, expression, and characterization of the Anabaena thioredoxin gene in Escherichia coli. J Bacteriol. 1986 Dec;168(3):1258–1264. doi: 10.1128/jb.168.3.1258-1264.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C. J., Gleason F. K., Jacobson B. A., Fuchs J. A. Characterization of Escherichia coli-Anabaena sp. hybrid thioredoxins. Biochemistry. 1988 Mar 8;27(5):1401–1408. doi: 10.1021/bi00405a002. [DOI] [PubMed] [Google Scholar]
- Marcus F., Moberly L., Latshaw S. P. Comparative amino acid sequence of fructose-1,6-bisphosphatases: identification of a region unique to the light-regulated chloroplast enzyme. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5379–5383. doi: 10.1073/pnas.85.15.5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlan S. C., Hogenkamp H. P., Eccleston E. D., Howard J. B., Fuchs J. A. Purification, characterization and revised amino acid sequence of a second thioredoxin from Corynebacterium nephridii. Eur J Biochem. 1989 Feb 1;179(2):389–398. doi: 10.1111/j.1432-1033.1989.tb14565.x. [DOI] [PubMed] [Google Scholar]
- Muller E. G., Buchanan B. B. Thioredoxin is essential for photosynthetic growth. The thioredoxin m gene of Anacystis nidulans. J Biol Chem. 1989 Mar 5;264(7):4008–4014. [PubMed] [Google Scholar]
- Muller E. G. Thioredoxin deficiency in yeast prolongs S phase and shortens the G1 interval of the cell cycle. J Biol Chem. 1991 May 15;266(14):9194–9202. [PubMed] [Google Scholar]
- Nikkola M., Gleason F. K., Saarinen M., Joelson T., Björnberg O., Eklund H. A putative glutathione-binding site in T4 glutaredoxin investigated by site-directed mutagenesis. J Biol Chem. 1991 Aug 25;266(24):16105–16112. [PubMed] [Google Scholar]
- Pigiet V. P., Conley R. R. Purification of thioredoxin, thioredoxin reductase, and glutathione reductase by affinity chromatography. J Biol Chem. 1977 Sep 25;252(18):6367–6372. [PubMed] [Google Scholar]
- Porter M. A., Stringer C. D., Hartman F. C. Characterization of the regulatory thioredoxin site of phosphoribulokinase. J Biol Chem. 1988 Jan 5;263(1):123–129. [PubMed] [Google Scholar]
- Scheibe R. Redox-modulation of chloroplast enzymes : a common principle for individual control. Plant Physiol. 1991 May;96(1):1–3. doi: 10.1104/pp.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabý I., Holmgren A. Structure and enzymatic functions of thioredoxin refolded by complementation of two tryptic peptide fragments. Biochemistry. 1979 Dec 11;18(25):5584–5591. doi: 10.1021/bi00592a010. [DOI] [PubMed] [Google Scholar]
- Whittaker M. M., Gleason F. K. Isolation and characterization of thioredoxin f from the filamentous cyanobacterium, Anabaena sp. 7119. J Biol Chem. 1984 Nov 25;259(22):14088–14093. [PubMed] [Google Scholar]
- Yang Y. F., Wells W. W. Identification and characterization of the functional amino acids at the active center of pig liver thioltransferase by site-directed mutagenesis. J Biol Chem. 1991 Jul 5;266(19):12759–12765. [PubMed] [Google Scholar]