Abstract
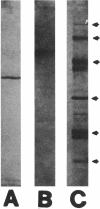
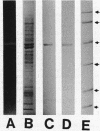
An intracellular form of calcium ion-dependent transglutaminase (R-glutaminylpeptide:amine gamma-glutaminyltransferase, EC 2.3.2.13) was purified 818-fold to apparent homogeneity from acetone powder preparations of spherules of the acellular slime mold Physarum polycephalum. The enzyme was purified by combined methods of precipitation with 15% (wt/vol) polyethylene glycol, DEAE-cellulose chromatography, and isoelectric focusing in a pH 5 to 7 gradient. The isoelectric point of the enzyme was 6.1. The molecular mass of the denatured enzyme was estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis to be 39.6 kDa. A molecular weight of 77,000 was found by gel filtration of the native enzyme on a Superose 12 fast protein liquid chromatography column, indicating that the native functional protein is a dimer. The purified transglutaminase catalyzed the incorporation of [14C]putrescine into protein substrates including casein, N,N'-dimethylcasein, actin purified from P. polycephalum, and actin purified from bovine muscle. Actin was the preferred substrate for the enzyme, both as a purified protein and in crude extracts prepared from P. polycephalum. With N,N'-dimethylcasein as the amine acceptor substrate, [14C]putrescine, [14C]spermidine, and [14C]spermine were all effective amine donor substrates with Km values of 49, 21.4, and 31.7 microM, respectively. All three of these polyamines demonstrated strong substrate inhibition of the enzyme activity between 100 and 200 microM. Upon starvation induced by depletion of a carbon source for growth, the specific activity of this enzyme increased sixfold during the differentiation of P. polycephalum microplasmodia to spherules. This suggests a role for transglutaminase in the construction of spherules, which have the capacity to survive starvation and dessication.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry E. L., Mosher D. F. Factor XIII cross-linking of fibronectin at cellular matrix assembly sites. J Biol Chem. 1988 Jul 25;263(21):10464–10469. [PubMed] [Google Scholar]
- Buxman M. M., Wuepper K. D. Isolation, purification and characterization of bovine epidermal transglutaminase. Biochim Biophys Acta. 1976 Dec 8;452(2):356–369. doi: 10.1016/0005-2744(76)90185-6. [DOI] [PubMed] [Google Scholar]
- Chin B., Bernstein I. A. Adenosine triphosphate and synchronous mitosis in Physarum polycephalum. J Bacteriol. 1968 Aug;96(2):330–337. doi: 10.1128/jb.96.2.330-337.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. I., Folk J. E. Transglutaminase from hair follicle of guinea pig (crosslinking-fibrin-glutamyllysine-isoenzymes-purified enzyme). Proc Natl Acad Sci U S A. 1972 Feb;69(2):303–307. doi: 10.1073/pnas.69.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. I., Lewis M. S., Folk J. E. Relationships of the catalytic properties of human plasma and platelet transglutaminases (activated blood coagulation factor XIII) to their subunit structures. J Biol Chem. 1974 Feb 10;249(3):940–950. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Davies P. J., Davies D. R., Levitzki A., Maxfield F. R., Milhaud P., Willingham M. C., Pastan I. H. Transglutaminase is essential in receptor-mediated endocytosis of alpha 2-macroglobulin and polypeptide hormones. Nature. 1980 Jan 10;283(5743):162–167. doi: 10.1038/283162a0. [DOI] [PubMed] [Google Scholar]
- Folk J. E., Cole P. W. Mechanism of action of guinea pig liver transglutaminase. I. Purification and properties of the enzyme: identification of a functional cysteine essential for activity. J Biol Chem. 1966 Dec 10;241(23):5518–5525. [PubMed] [Google Scholar]
- Folk J. E. Transglutaminases. Annu Rev Biochem. 1980;49:517–531. doi: 10.1146/annurev.bi.49.070180.002505. [DOI] [PubMed] [Google Scholar]
- Goodman E. M., Sauer H. W., Sauer L., Rusch H. P. Polyphosphate and other phosphorus compounds during growth and differentiation of Physarum polycephalum. Can J Microbiol. 1969 Nov;15(11):1325–1331. doi: 10.1139/m69-240. [DOI] [PubMed] [Google Scholar]
- Hatano S., Oosawa F. Extraction of an actin-like protein from the plasmodium of a myxomycete and its interaction with myosin A from rabbit striated muscle. J Cell Physiol. 1966 Oct;68(2):197–202. doi: 10.1002/jcp.1040680214. [DOI] [PubMed] [Google Scholar]
- Hatano S., Oosawa F. Isolation and characterization of plasmodium actin. Biochim Biophys Acta. 1966 Oct 31;127(2):488–498. doi: 10.1016/0304-4165(66)90402-8. [DOI] [PubMed] [Google Scholar]
- Hiatt W. R., Whiteley H. R. Activity of uridine diphosphate N-acetylglucosamine-4-epimerase during spherulation of Physarum polycephalum. J Bacteriol. 1974 May;118(2):761–763. doi: 10.1128/jb.118.2.761-763.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N. Cross-linker system between neurofilaments, microtubules, and membranous organelles in frog axons revealed by the quick-freeze, deep-etching method. J Cell Biol. 1982 Jul;94(1):129–142. doi: 10.1083/jcb.94.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose A., Bottenus R. E., Davie E. W. Structure of transglutaminases. J Biol Chem. 1990 Aug 15;265(23):13411–13414. [PubMed] [Google Scholar]
- Ichinose A., Davie E. W. Characterization of the gene for the a subunit of human factor XIII (plasma transglutaminase), a blood coagulation factor. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5829–5833. doi: 10.1073/pnas.85.16.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikura K., Nasu T., Yokota H., Tsuchiya Y., Sasaki R., Chiba H. Amino acid sequence of guinea pig liver transglutaminase from its cDNA sequence. Biochemistry. 1988 Apr 19;27(8):2898–2905. doi: 10.1021/bi00408a035. [DOI] [PubMed] [Google Scholar]
- Kim H. C., Idler W. W., Kim I. G., Han J. H., Chung S. I., Steinert P. M. The complete amino acid sequence of the human transglutaminase K enzyme deduced from the nucleic acid sequences of cDNA clones. J Biol Chem. 1991 Jan 5;266(1):536–539. [PubMed] [Google Scholar]
- Kuehn G. D. Uridine diphosphoglucose pyrophosphorylase activity and differentiation in the cellular slime mold Physarum polycephaluno. J Bacteriol. 1974 Dec;120(3):1151–1157. doi: 10.1128/jb.120.3.1151-1157.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leary J. J., Brigati D. J., Ward D. C. Rapid and sensitive colorimetric method for visualizing biotin-labeled DNA probes hybridized to DNA or RNA immobilized on nitrocellulose: Bio-blots. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4045–4049. doi: 10.1073/pnas.80.13.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewy A. G., Matacic S. S. Modulation of the epsilon-(gamma-glutamic)lysine cross-link in cellular proteins. I. In vivo and in vitro studies. Biochim Biophys Acta. 1981 Mar 27;668(1):167–176. doi: 10.1016/0005-2795(81)90160-4. [DOI] [PubMed] [Google Scholar]
- Lorand L., Campbell-Wilkes L. K., Cooperstein L. A filter paper assay for transamidating enzymes using radioactive amine substrates. Anal Biochem. 1972 Dec;50(2):623–631. doi: 10.1016/0003-2697(72)90074-7. [DOI] [PubMed] [Google Scholar]
- Lorand L., Rule N. G., Ong H. H., Furlanetto R., Jacobsen A., Downey J., Oner N., Bruner-Lorand J. Amine specificity in transpeptidation. Inhibition of fibrin cross-linking. Biochemistry. 1968 Mar;7(3):1214–1223. doi: 10.1021/bi00843a043. [DOI] [PubMed] [Google Scholar]
- Lorand L., Siefring G. E., Jr, Tong Y. S., Bruner-Lorand J., Gray A. J., Jr Dansylcadaverine specific staining for transamidating enzymes. Anal Biochem. 1979 Mar;93(2):453–458. doi: 10.1016/s0003-2697(79)80178-5. [DOI] [PubMed] [Google Scholar]
- Lorand L., Weissmann L. B., Epel D. L., Bruner-Lorand J. Role of the intrinsic transglutaminase in the Ca2+-mediated crosslinking of erythrocyte proteins. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4479–4481. doi: 10.1073/pnas.73.12.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margosiak S. A., Dharma A., Bruce-Carver M. R., Gonzales A. P., Louie D., Kuehn G. D. Identification of the Large Subunit of Ribulose 1,5-Bisphosphate Carboxylase/Oxygenase as a Substrate for Transglutaminase in Medicago sativa L. (Alfalfa). Plant Physiol. 1990 Jan;92(1):88–96. doi: 10.1104/pp.92.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K., Kimura S., Ohashi K., Kuwano Y. Connectin, an elastic protein of muscle. Identification of "titin" with connectin. J Biochem. 1981 Mar;89(3):701–709. doi: 10.1093/oxfordjournals.jbchem.a133249. [DOI] [PubMed] [Google Scholar]
- Michel S., Démarchez M. Localization and in vivo activity of epidermal transglutaminase. J Invest Dermatol. 1988 Apr;90(4):472–474. doi: 10.1111/1523-1747.ep12460922. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Ozaki K., Maruyama K. Connectin, an elastic protein of muscle. A connectin-like protein from the plasmodium Physarum polycephalum. J Biochem. 1980 Sep;88(3):883–888. doi: 10.1093/oxfordjournals.jbchem.a133043. [DOI] [PubMed] [Google Scholar]
- Peterson L. L., Wuepper K. D. Epidermal and hair follicle transglutaminases and crosslinking in skin. Mol Cell Biochem. 1984;58(1-2):99–111. doi: 10.1007/BF00240609. [DOI] [PubMed] [Google Scholar]
- Polanshek M. M., Blomquist J. C., Evans T. E., Rusch H. P. Aminopeptidases of Physarum polycephalum during growth and differentiation. Arch Biochem Biophys. 1978 Sep;190(1):261–269. doi: 10.1016/0003-9861(78)90275-8. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Green H. The cornified envelope of terminally differentiated human epidermal keratinocytes consists of cross-linked protein. Cell. 1977 Jun;11(2):417–422. doi: 10.1016/0092-8674(77)90059-9. [DOI] [PubMed] [Google Scholar]
- Rusch H. P. Some biochemical events in the growth cycles of Physarum polycephalum. Fed Proc. 1969 Nov-Dec;28(6):1761–1770. [PubMed] [Google Scholar]
- Sane D. C., Moser T. L., Pippen A. M., Parker C. J., Achyuthan K. E., Greenberg C. S. Vitronectin is a substrate for transglutaminases. Biochem Biophys Res Commun. 1988 Nov 30;157(1):115–120. doi: 10.1016/s0006-291x(88)80020-2. [DOI] [PubMed] [Google Scholar]
- Schliwa M., van Blerkom J. Structural interaction of cytoskeletal components. J Cell Biol. 1981 Jul;90(1):222–235. doi: 10.1083/jcb.90.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zettergren J. G., Peterson L. L., Wuepper K. D. Keratolinin: the soluble substrate of epidermal transglutaminase from human and bovine tissue. Proc Natl Acad Sci U S A. 1984 Jan;81(1):238–242. doi: 10.1073/pnas.81.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]