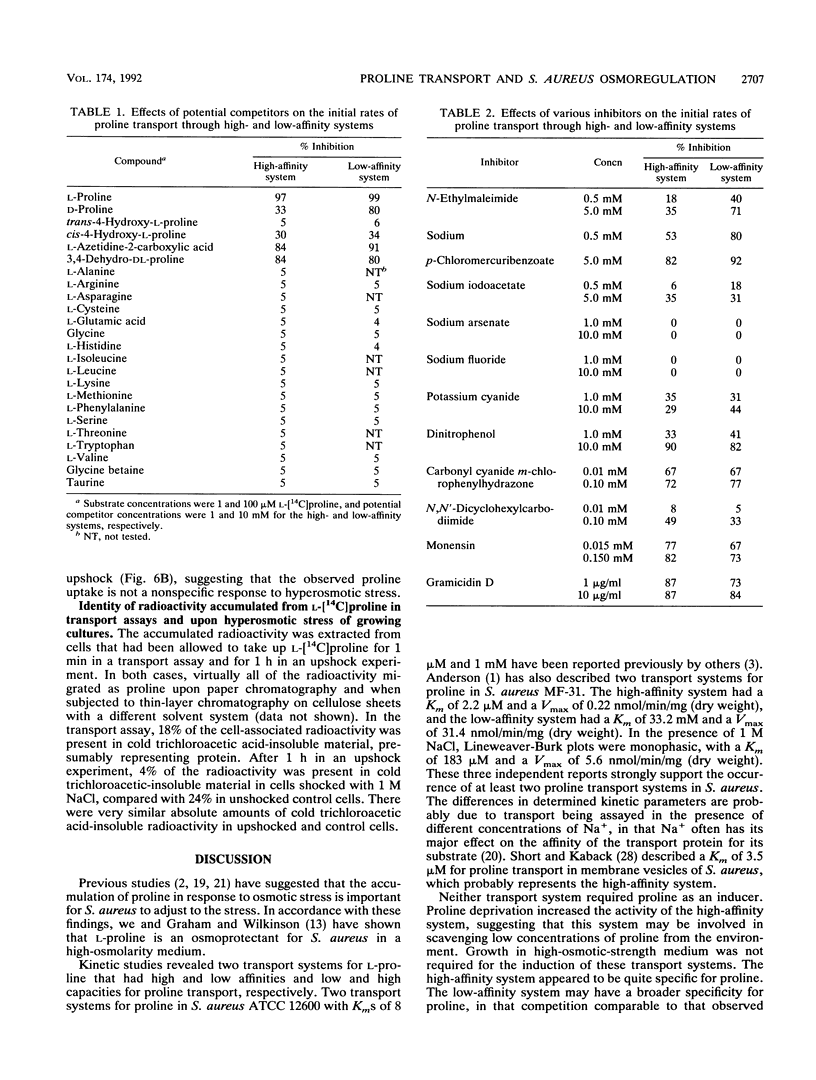
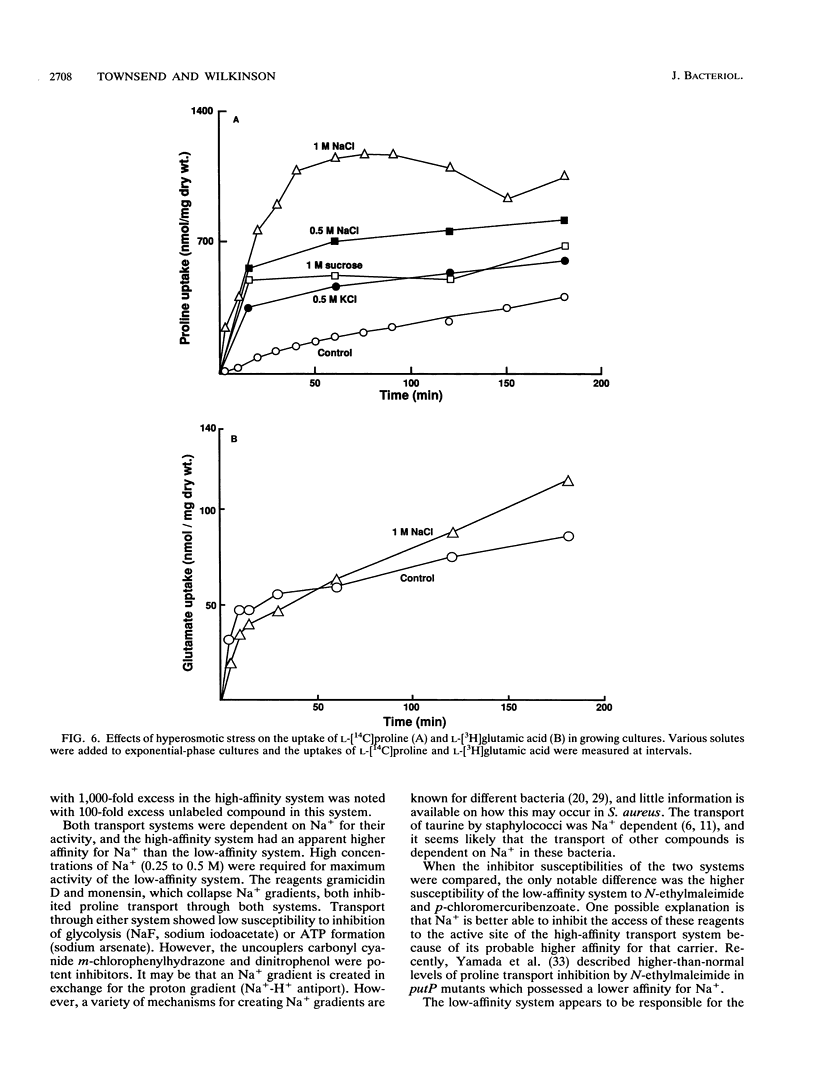
Abstract
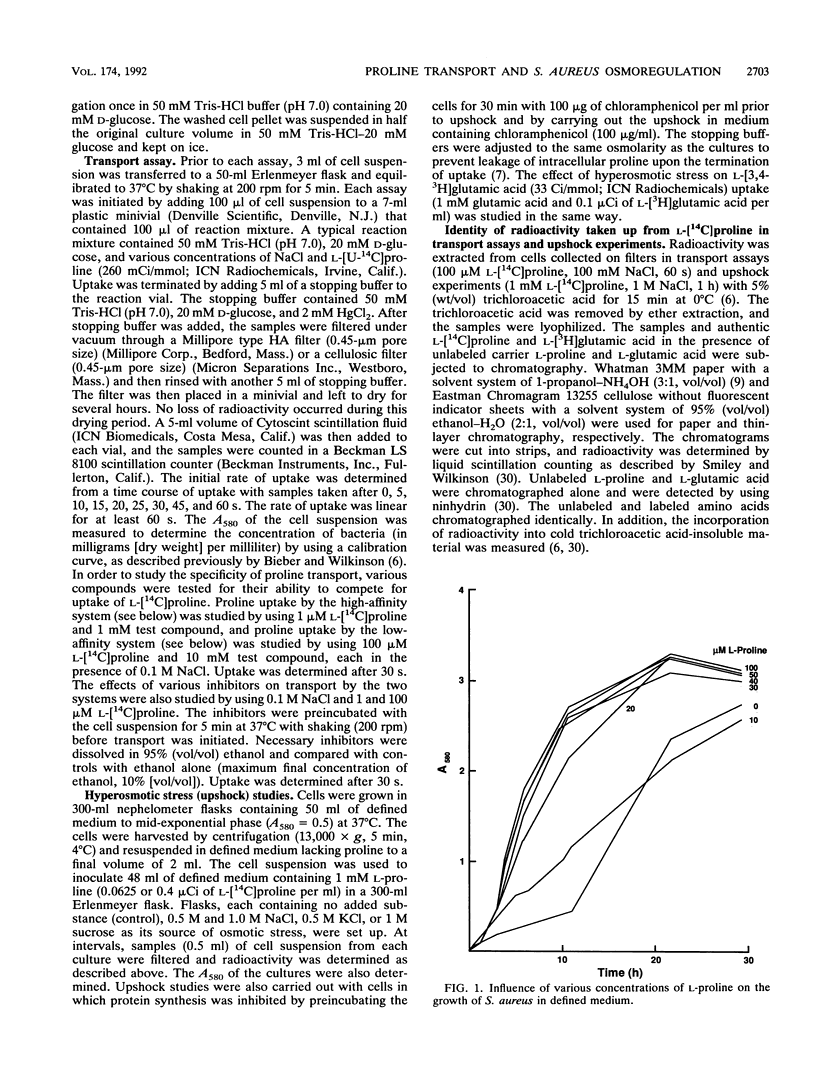
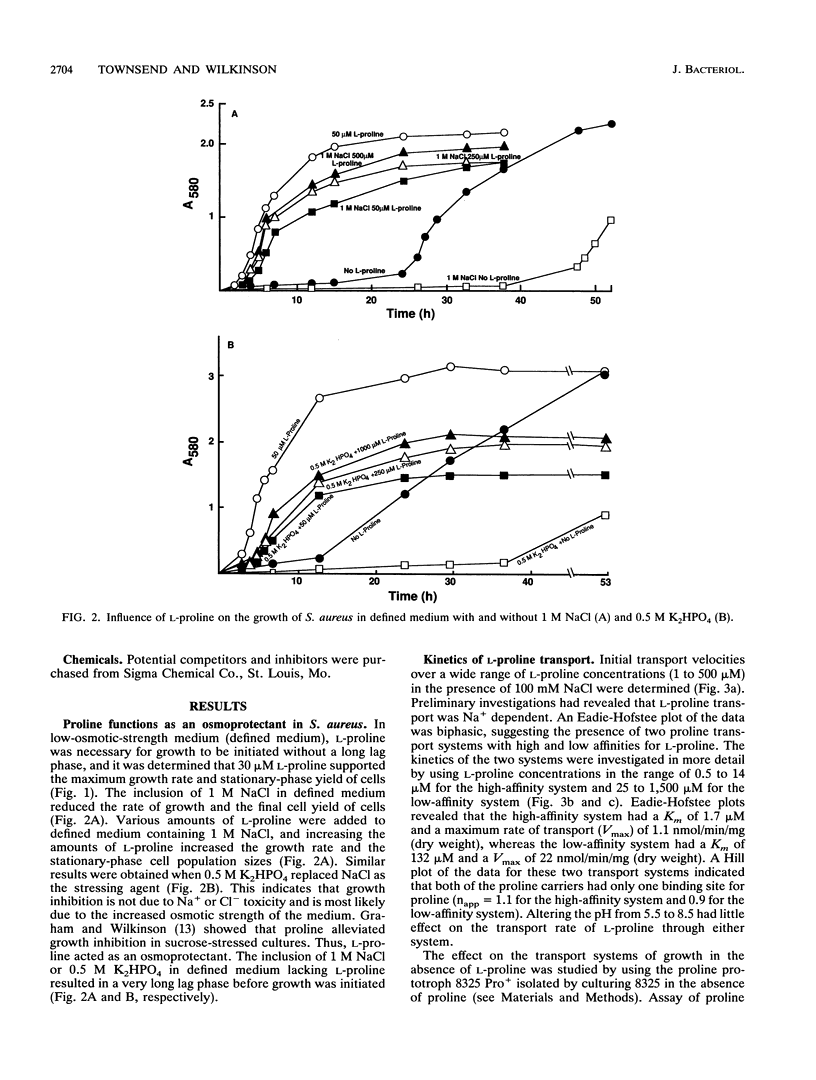
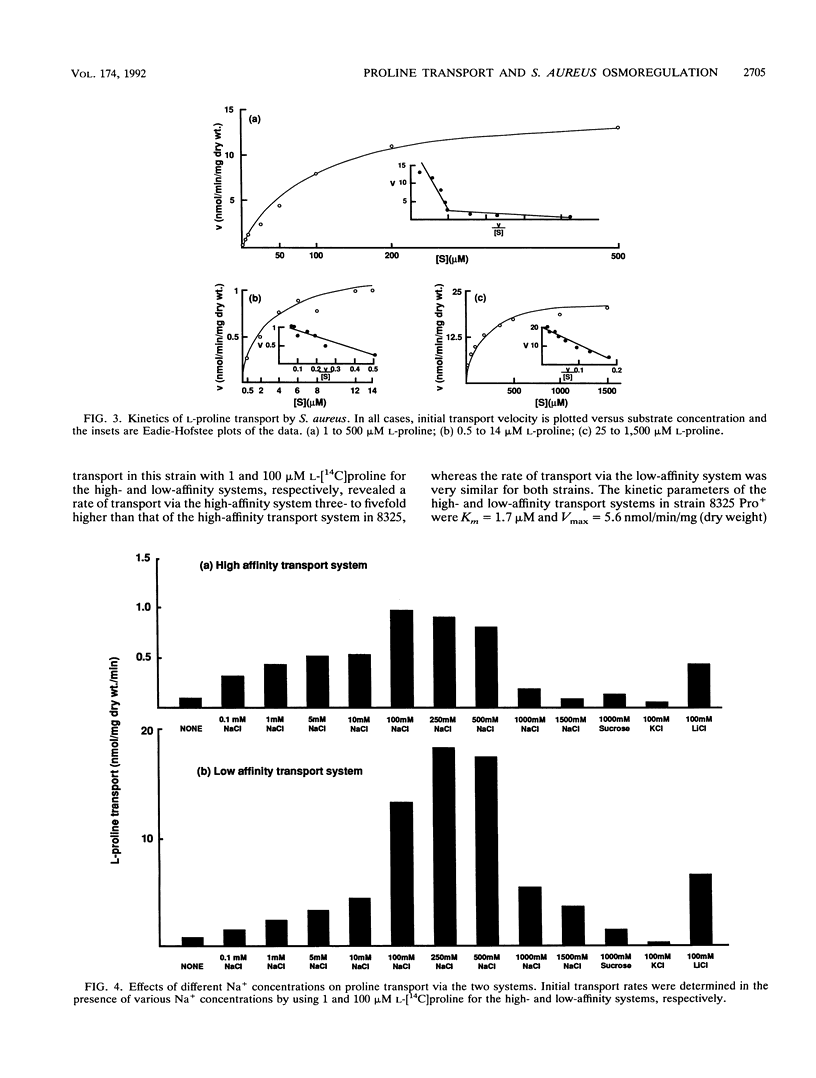
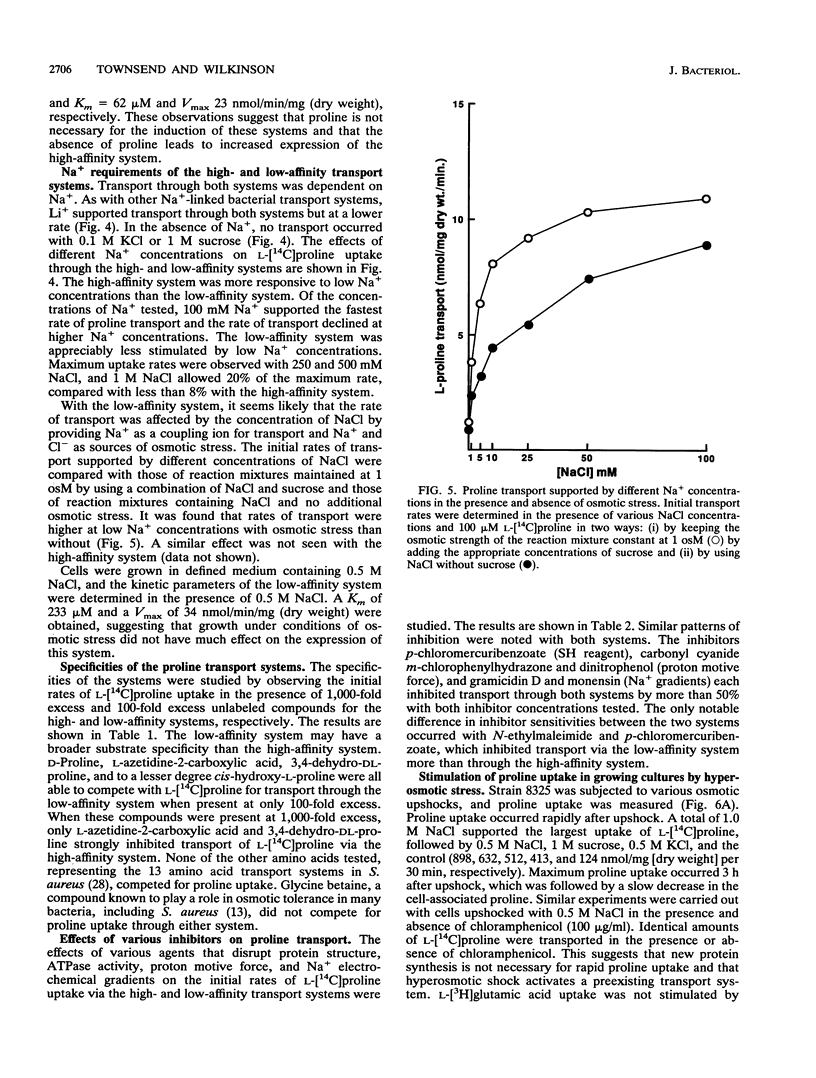
L-Proline enhanced the growth of Staphylococcus aureus in high-osmotic-strength medium, i.e., it acted as an osmoprotectant. Study of the kinetics of L-[14C]proline uptake by S. aureus NCTC 8325 revealed high-affinity (Km = 1.7 microM; maximum rate of transport [Vmax] = 1.1 nmol/min/mg [dry weight]) and low-affinity (Km = 132 microM; Vmax = 22 nmol/min/mg [dry weight]) transport systems. Both systems were present in a proline prototrophic variant grown in the absence of proline, although the Vmax of the high-affinity system was three to five times higher than that of the high-affinity system in strain 8325. Both systems were dependent on Na+ for activity, and the high-affinity system was stimulated by lower concentrations of Na+ more than the low-affinity system. The proline transport activity of the low-affinity system was stimulated by increased osmotic strength. The high-affinity system was highly specific for L-proline, whereas the low-affinity system showed a broader substrate specificity. Glycine betaine did not compete with proline for uptake through either system. Inhibitor studies confirmed that proline uptake occurred via Na(+)-dependent systems and suggested the involvement of the proton motive force in creating an Na+ gradient. Hyperosmotic stress (upshock) of growing cultures led to a rapid and large uptake of L-[14C]proline that was not dependent on new protein synthesis. It is suggested that the low-affinity system is involved in adjusting to increased environmental osmolarity and that the high-affinity system may be involved in scavenging low concentrations of proline.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. B., Witter L. D. Glutamine and proline accumulation by Staphylococcus aureus with reduction in water activity. Appl Environ Microbiol. 1982 Jun;43(6):1501–1503. doi: 10.1128/aem.43.6.1501-1503.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron A., Jung J. U., Villarejo M. Purification and characterization of a glycine betaine binding protein from Escherichia coli. J Biol Chem. 1987 Aug 25;262(24):11841–11846. [PubMed] [Google Scholar]
- Bascarán V., Hardisson C., Braña A. F. Proline uptake in Streptomyces clavuligerus. FEMS Microbiol Lett. 1990 May;57(1-2):27–30. doi: 10.1016/0378-1097(90)90407-h. [DOI] [PubMed] [Google Scholar]
- Bieber E. J., Wilkinson B. J. Sodium-dependent uptake of taurine in encapsulated Staphylococcus aureus strain M. Biochim Biophys Acta. 1984 Mar 14;770(2):127–135. doi: 10.1016/0005-2736(84)90121-4. [DOI] [PubMed] [Google Scholar]
- CHRISTIAN J. H., WALTHO J. A. THE COMPOSITION OF STAPHYLOCOCCUS AUREUS IN RELATION TO THE WATER ACTIVITY OF THE GROWTH MEDIUM. J Gen Microbiol. 1964 May;35:205–213. doi: 10.1099/00221287-35-2-205. [DOI] [PubMed] [Google Scholar]
- Csonka L. N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989 Mar;53(1):121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutch C. E., Hasler J. M., Houston R. M., Sharma M., Stone V. J. Nonspecific inhibition of proline dehydrogenase synthesis in Escherichia coli during osmotic stress. Can J Microbiol. 1989 Aug;35(8):779–785. doi: 10.1139/m89-130. [DOI] [PubMed] [Google Scholar]
- Giehl T. J., Qoronfleh M. W., Wilkinson B. J. Transport, nutritional and metabolic studies of taurine in staphylococci. J Gen Microbiol. 1987 Apr;133(4):849–856. doi: 10.1099/00221287-133-4-849. [DOI] [PubMed] [Google Scholar]
- Graham J. E., Wilkinson B. J. Staphylococcus aureus osmoregulation: roles for choline, glycine betaine, proline, and taurine. J Bacteriol. 1992 Apr;174(8):2711–2716. doi: 10.1128/jb.174.8.2711-2716.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iandolo J. J. Genetic analysis of extracellular toxins of Staphylococcus aureus. Annu Rev Microbiol. 1989;43:375–402. doi: 10.1146/annurev.mi.43.100189.002111. [DOI] [PubMed] [Google Scholar]
- Jovanovich S. B., Record M. T., Jr, Burgess R. R. In an Escherichia coli coupled transcription-translation system, expression of the osmoregulated gene proU is stimulated at elevated potassium concentrations and by an extract from cells grown at high osmolality. J Biol Chem. 1989 May 15;264(14):7821–7825. [PubMed] [Google Scholar]
- Koujima I., Hayashi H., Tomochika K., Okabe A., Kanemasa Y. Adaptational change in proline and water content of Staphylococcus aureus after alteration of environmental salt concentration. Appl Environ Microbiol. 1978 Mar;35(3):467–470. doi: 10.1128/aem.35.3.467-470.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Measures J. C. Role of amino acids in osmoregulation of non-halophilic bacteria. Nature. 1975 Oct 2;257(5525):398–400. doi: 10.1038/257398a0. [DOI] [PubMed] [Google Scholar]
- Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967 Sep;33(1):155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- Ordal G. W., Villani D. P., Nicholas R. A., Hamel F. G. Independence of proline chemotaxis and transport in Bacillus subtilis. J Biol Chem. 1978 Jul 25;253(14):4916–4919. [PubMed] [Google Scholar]
- Pattee P. A., Neveln D. S. Transformation analysis of three linkage groups in Staphylococcus aureus. J Bacteriol. 1975 Oct;124(1):201–211. doi: 10.1128/jb.124.1.201-211.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez R. M., Prince W. S., Bremer E., Villarejo M. In vitro reconstitution of osmoregulated expression of proU of Escherichia coli. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1153–1157. doi: 10.1073/pnas.86.4.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT W. J. Water relations of Staphylococcus aureus at 30 degrees C. Aust J Biol Sci. 1953 Nov;6(4):549–564. [PubMed] [Google Scholar]
- Short S. A., Kaback H. R. Amino acid transport and staphylococcal membrane vesicles. Ann N Y Acad Sci. 1974 Jul 31;236(0):124–143. doi: 10.1111/j.1749-6632.1974.tb41487.x. [DOI] [PubMed] [Google Scholar]
- Smiley D. W., Wilkinson B. J. Survey of taurine uptake and metabolism in Staphylococcus aureus. J Gen Microbiol. 1983 Aug;129(8):2421–2428. doi: 10.1099/00221287-129-8-2421. [DOI] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. M. Proline porters effect the utilization of proline as nutrient or osmoprotectant for bacteria. J Membr Biol. 1988 Dec;106(3):183–202. doi: 10.1007/BF01872157. [DOI] [PubMed] [Google Scholar]
- Yamato I., Ohsawa M., Anraku Y. Defective cation-coupling mutants of Escherichia coli Na+/proline symport carrier. Characterization and localization of mutations. J Biol Chem. 1990 Feb 15;265(5):2450–2455. [PubMed] [Google Scholar]


