Abstract
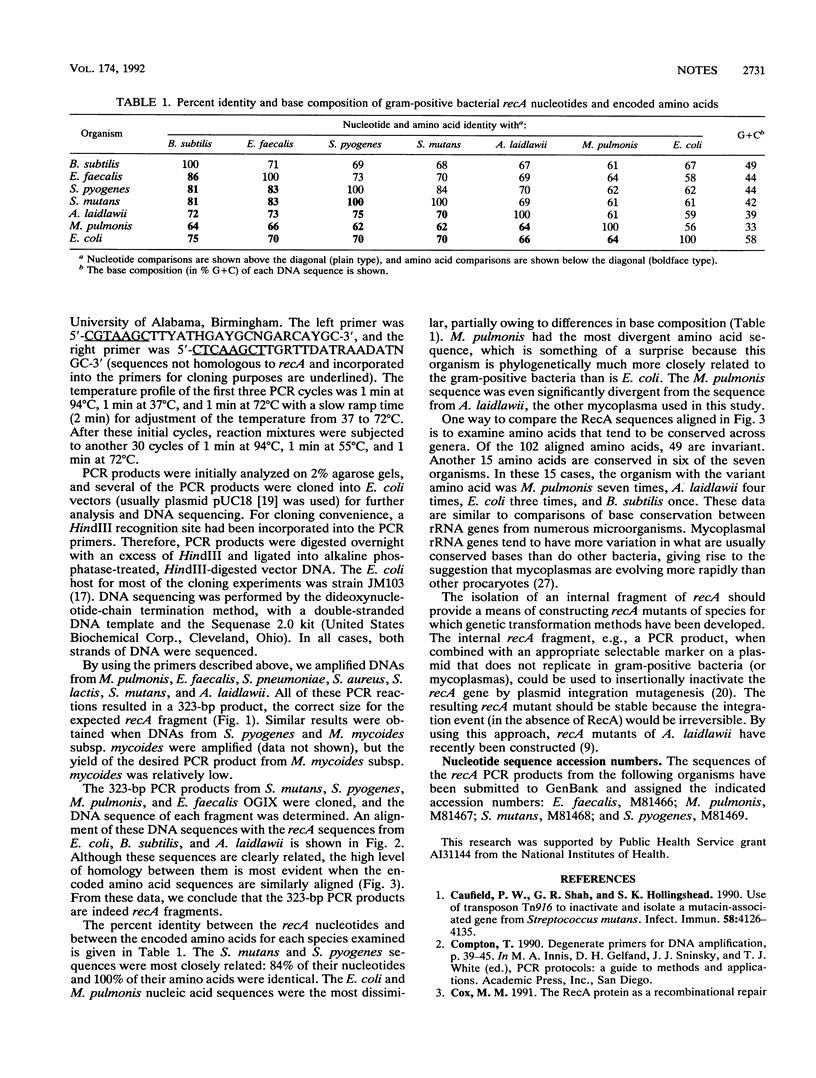
RecA protein in gram-negative bacteria, especially in Escherichia coli, has been extensively studied, but little is known about this key enzyme in other procaryotes. Described here are degenerate oligonucleotide primers that have been used to amplify by the polymerase chain reaction (PCR) recA sequences from several gram-positive bacteria and mycoplasmas. The DNA sequences of recA PCR products from Streptococcus pyogenes, Streptococcus mutans, Enterococcus faecalis, and Mycoplasma pulmonis were determined and compared. These data indicate that the M. pulmonis recA gene has diverged significantly from recA genes of other eubacteria. It should be possible to use cloned recA PCR products to construct recA mutants, thereby providing the means of elucidating homologous genetic recombination and DNA repair activities in these organisms.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caufield P. W., Shah G. R., Hollingshead S. K. Use of transposon Tn916 to inactivate and isolate a mutacin-associated gene from Streptococcus mutans. Infect Immun. 1990 Dec;58(12):4126–4135. doi: 10.1128/iai.58.12.4126-4135.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox M. M. The RecA protein as a recombinational repair system. Mol Microbiol. 1991 Jun;5(6):1295–1299. doi: 10.1111/j.1365-2958.1991.tb00775.x. [DOI] [PubMed] [Google Scholar]
- Dybvig K., Alderete J. Transformation of Mycoplasma pulmonis and Mycoplasma hyorhinis: transposition of Tn916 and formation of cointegrate structures. Plasmid. 1988 Jul;20(1):33–41. doi: 10.1016/0147-619x(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Dybvig K., Cassell G. H. Transposition of gram-positive transposon Tn916 in Acholeplasma laidlawii and Mycoplasma pulmonis. Science. 1987 Mar 13;235(4794):1392–1394. doi: 10.1126/science.3029869. [DOI] [PubMed] [Google Scholar]
- Dybvig K., Khaled M. Isolation of a second cryptic plasmid from Mycoplasma mycoides subsp. mycoides. Plasmid. 1990 Sep;24(2):153–155. doi: 10.1016/0147-619x(90)90018-8. [DOI] [PubMed] [Google Scholar]
- Dybvig K., Simecka J. W., Watson H. L., Cassell G. H. High-frequency variation in Mycoplasma pulmonis colony size. J Bacteriol. 1989 Sep;171(9):5165–5168. doi: 10.1128/jb.171.9.5165-5168.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybvig K., Woodard A. Cloning and DNA sequence of a mycoplasmal recA gene. J Bacteriol. 1992 Feb;174(3):778–784. doi: 10.1128/jb.174.3.778-784.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasson M. J. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983 Apr;154(1):1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ike Y., Craig R. A., White B. A., Yagi Y., Clewell D. B. Modification of Streptococcus faecalis sex pheromones after acquisition of plasmid DNA. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5369–5373. doi: 10.1073/pnas.80.17.5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Love P. E., Yasbin R. E. Induction of the Bacillus subtilis SOS-like response by Escherichia coli RecA protein. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5204–5208. doi: 10.1073/pnas.83.14.5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett C. M., Jr, Love P. E., Yasbin R. E., Roberts J. W. SOS-like induction in Bacillus subtilis: induction of the RecA protein analog and a damage-inducible operon by DNA damage in Rec+ and DNA repair-deficient strains. J Bacteriol. 1988 Apr;170(4):1467–1474. doi: 10.1128/jb.170.4.1467-1474.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahairas G. G., Minion F. C. Transformation of Mycoplasma pulmonis: demonstration of homologous recombination, introduction of cloned genes, and preliminary description of an integrating shuttle system. J Bacteriol. 1989 Apr;171(4):1775–1780. doi: 10.1128/jb.171.4.1775-1780.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniloff J. Evolution of wall-less prokaryotes. Annu Rev Microbiol. 1983;37:477–499. doi: 10.1146/annurev.mi.37.100183.002401. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. V., Kokjohn T. A. General microbiology of recA: environmental and evolutionary significance. Annu Rev Microbiol. 1990;44:365–394. doi: 10.1146/annurev.mi.44.100190.002053. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Piggot P. J., Curtis C. A., de Lencastre H. Use of integrational plasmid vectors to demonstrate the polycistronic nature of a transcriptional unit (spoIIA) required for sporulation of Bacillus subtilis. J Gen Microbiol. 1984 Aug;130(8):2123–2136. doi: 10.1099/00221287-130-8-2123. [DOI] [PubMed] [Google Scholar]
- Radding C. M. Homologous pairing and strand exchange in genetic recombination. Annu Rev Genet. 1982;16:405–437. doi: 10.1146/annurev.ge.16.120182.002201. [DOI] [PubMed] [Google Scholar]
- Sancar A., Stachelek C., Konigsberg W., Rupp W. D. Sequences of the recA gene and protein. Proc Natl Acad Sci U S A. 1980 May;77(5):2611–2615. doi: 10.1073/pnas.77.5.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. R., Fischetti V. A. Expression of streptococcal M protein in Escherichia coli. Science. 1983 Aug 19;221(4612):758–760. doi: 10.1126/science.6192499. [DOI] [PubMed] [Google Scholar]
- Sladek T. L., Nowak J. A., Maniloff J. Mycoplasma restriction: identification of a new type of restriction specificity for DNA containing 5-methylcytosine. J Bacteriol. 1986 Jan;165(1):219–225. doi: 10.1128/jb.165.1.219-225.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranathan M. C., Bayles K. W., Yasbin R. E. The nucleotide sequence of the recE+ gene of Bacillus subtilis. Nucleic Acids Res. 1990 Jul 25;18(14):4249–4249. doi: 10.1093/nar/18.14.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]



