Abstract
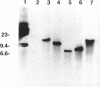
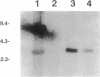
Broad-host-range IncP and IncQ plasmids have been transferred to the aerobic magnetic bacterium Aquaspirillum sp. strain AMB-1. Conjugal matings with Escherichia coli S17-1 allowed high-frequency transfer of the RK2 derivative pRK415 (4.5 x 10(-3) transconjugant per recipient cell) and the RSF1010 derivative pKT230 (3.0 x 10(-3) transconjugant per recipient). These plasmids successfully formed autonomous replicons in transconjugants and could be isolated and transformed back into E. coli, illustrating their potential as shuttle vectors. A mobilizable plasmid containing transposon Tn5 was transferred to Aquaspirillum sp. strain AMB-1 and also to the obligately microaerophilic magnetic bacterium Aquaspirillum magnetotacticum MS-1. Five nonmagnetic kanamycin-resistant mutants of Aquaspirillum sp. strain AMB-1 in which Tn5 was shown to be integrated into the chromosome were obtained. Different genomic fragments containing the mutagenized regions were cloned into E. coli. Two genomic fragments were restriction mapped, and the site of Tn5 insertion was determined. They were shown to be identical, although derived from independent transposon insertions. One of these clones was found to hybridize strongly to regions of the A. magnetotacticum MS-1 chromosome. This is the first report of gene transfer in a magnetic bacterium.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagdasarian M., Lurz R., Rückert B., Franklin F. C., Bagdasarian M. M., Frey J., Timmis K. N. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981 Dec;16(1-3):237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- Blakemore R. P. Magnetotactic bacteria. Annu Rev Microbiol. 1982;36:217–238. doi: 10.1146/annurev.mi.36.100182.001245. [DOI] [PubMed] [Google Scholar]
- Blakemore R. P., Maratea D., Wolfe R. S. Isolation and pure culture of a freshwater magnetic spirillum in chemically defined medium. J Bacteriol. 1979 Nov;140(2):720–729. doi: 10.1128/jb.140.2.720-729.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore R. Magnetotactic bacteria. Science. 1975 Oct 24;190(4212):377–379. doi: 10.1126/science.170679. [DOI] [PubMed] [Google Scholar]
- Eden P. A., Blakemore R. P. Electroporation and conjugal plasmid transfer to members of the genus Aquaspirillum. Arch Microbiol. 1991;155(5):449–452. doi: 10.1007/BF00244960. [DOI] [PubMed] [Google Scholar]
- Gould J. L., Kirschvink J. L., Deffeyes K. S. Bees have magnetic remanence. Science. 1978 Sep 15;201(4360):1026–1028. doi: 10.1126/science.201.4360.1026. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Kalmijn A. J. Electric and magnetic field detection in elasmobranch fishes. Science. 1982 Nov 26;218(4575):916–918. doi: 10.1126/science.7134985. [DOI] [PubMed] [Google Scholar]
- Keen N. T., Tamaki S., Kobayashi D., Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988 Oct 15;70(1):191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- Keeton W. T. Magnets interfere with pigeon homing. Proc Natl Acad Sci U S A. 1971 Jan;68(1):102–106. doi: 10.1073/pnas.68.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann S., Sparks N. H., Board R. G. Magnetotactic bacteria: microbiology, biomineralization, palaeomagnetism and biotechnology. Adv Microb Physiol. 1990;31:125–181. doi: 10.1016/s0065-2911(08)60121-6. [DOI] [PubMed] [Google Scholar]
- Matsunaga T. Applications of bacterial magnets. Trends Biotechnol. 1991 Mar;9(3):91–95. doi: 10.1016/0167-7799(91)90031-c. [DOI] [PubMed] [Google Scholar]
- Simon R., O'Connell M., Labes M., Pühler A. Plasmid vectors for the genetic analysis and manipulation of rhizobia and other gram-negative bacteria. Methods Enzymol. 1986;118:640–659. doi: 10.1016/0076-6879(86)18106-7. [DOI] [PubMed] [Google Scholar]
- Walcott C., Gould J. L., Kirschvink J. L. Pigeons have magnets. Science. 1979 Sep 7;205(4410):1027–1029. doi: 10.1126/science.472725. [DOI] [PubMed] [Google Scholar]
- Walcott C., Green R. P. Orientation of homing pigeons altered by a change in the direction of an applied magnetic field. Science. 1974 Apr 12;184(4133):180–182. doi: 10.1126/science.184.4133.180. [DOI] [PubMed] [Google Scholar]
- Walker M. M., Kirschvink J. L., Chang S. B., Dizon A. E. A Candidate Magnetic Sense Organ in the Yellowfin Tuna, Thunnus albacares. Science. 1984 May 18;224(4650):751–753. doi: 10.1126/science.224.4650.751. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]