Abstract
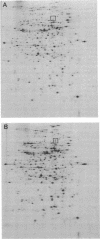
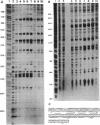
In Escherichia coli K-12, expression of the lysU gene is regulated by the lrp gene product, as indicated by an increase in the level of lysyl-tRNA synthetase activity and LysU protein in an lrp mutant. Comparison of the patterns of protein expression visualized by two-dimensional gel electrophoresis indicated that LysU is present at higher levels in an lrp strain than in its isogenic lrp+ parent. The purified lrp gene product was shown to bind to sites upstream of the lysU gene and to protect several sites against DNase I digestion. A region extending over 100 nucleotides, between 60 and 160 nucleotides upstream from the start of the lysU coding sequence, showed altered sensitivity to DNase I digestion in the presence of the Lrp protein. The extent of protected DNA suggests a complex interaction of Lrp protein and upstream lysU DNA.
Full text
PDF


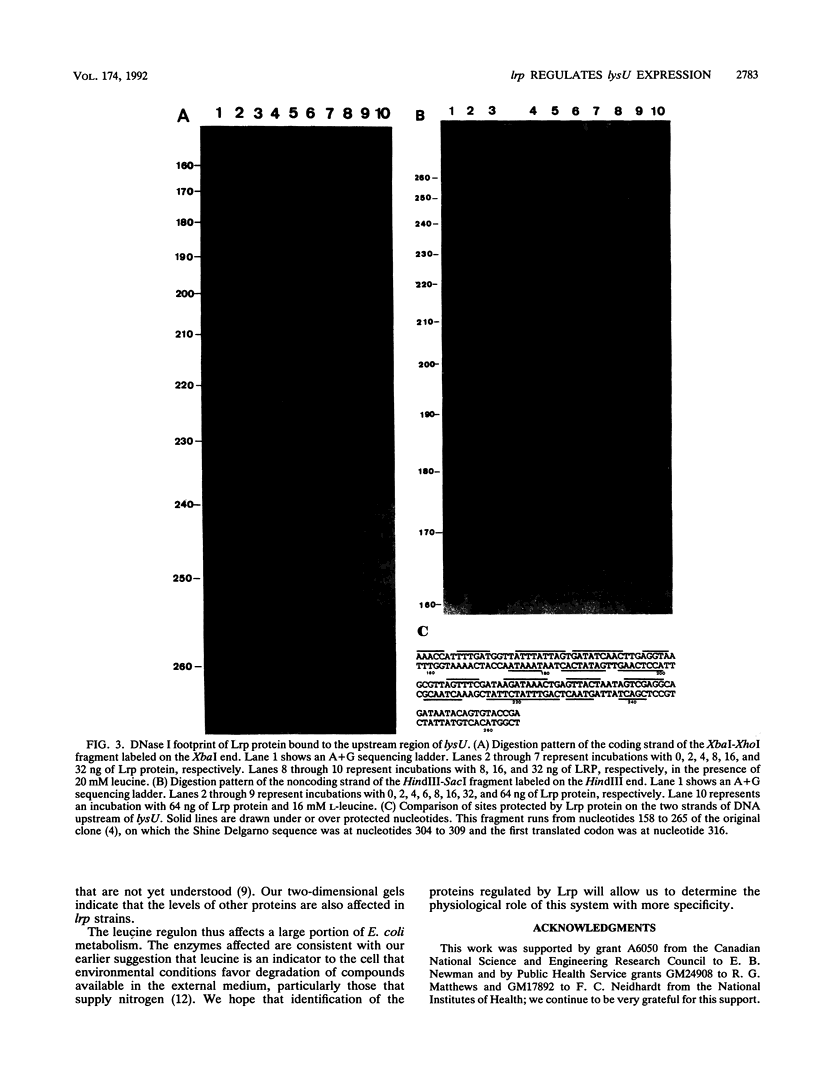

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews J. C., Blevins T. C., Short S. A. Regulation of peptide transport in Escherichia coli: induction of the trp-linked operon encoding the oligopeptide permease. J Bacteriol. 1986 Feb;165(2):428–433. doi: 10.1128/jb.165.2.428-433.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews J. C., Short S. A. opp-lac Operon fusions and transcriptional regulation of the Escherichia coli trp-linked oligopeptide permease. J Bacteriol. 1986 Feb;165(2):434–442. doi: 10.1128/jb.165.2.434-442.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal R. M., Reeh S., Pedersen S. Regulation of transcription factor rho and the alpha subunit of RNA polymerase in Escherichia coli B/r. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2285–2288. doi: 10.1073/pnas.73.7.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. L., Neidhardt F. C. Roles of the two lysyl-tRNA synthetases of Escherichia coli: analysis of nucleotide sequences and mutant behavior. J Bacteriol. 1990 Jun;172(6):3237–3243. doi: 10.1128/jb.172.6.3237-3243.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama H., Shimamoto T., Tsuda M., Tsuchiya T. Characterization of a novel L-serine transport system in Escherichia coli. J Bacteriol. 1988 May;170(5):2236–2239. doi: 10.1128/jb.170.5.2236-2239.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield I. N., Bloch P. L., Van Bogelen R. A., Neidhardt F. C. Multiple forms of lysyl-transfer ribonucleic acid synthetase in Escherichia coli. J Bacteriol. 1981 Apr;146(1):345–351. doi: 10.1128/jb.146.1.345-351.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield I. N., Tenreiro R., Vanbogelen R. A., Neidhardt F. C. Escherichia coli K-12 lysyl-tRNA synthetase mutant with a novel reversion pattern. J Bacteriol. 1984 May;158(2):615–620. doi: 10.1128/jb.158.2.615-620.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg S., Newman E. B. Studies on L-serine deaminase in Escherichia coli K-12. J Bacteriol. 1974 Apr;118(1):53–58. doi: 10.1128/jb.118.1.53-58.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews R. G., Neidhardt F. C. Abnormal induction of heat shock proteins in an Escherichia coli mutant deficient in adenosylmethionine synthetase activity. J Bacteriol. 1988 Apr;170(4):1582–1588. doi: 10.1128/jb.170.4.1582-1588.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews R. G., Neidhardt F. C. Elevated serine catabolism is associated with the heat shock response in Escherichia coli. J Bacteriol. 1989 May;171(5):2619–2625. doi: 10.1128/jb.171.5.2619-2625.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E. B., Kapoor V., Potter R. Role of L-threonine dehydrogenase in the catabolism of threonine and synthesis of glycine by Escherichia coli. J Bacteriol. 1976 Jun;126(3):1245–1249. doi: 10.1128/jb.126.3.1245-1249.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E. B., Miller B., Colebrook L. D., Walker C. A mutation in Escherichia coli K-12 results in a requirement for thiamine and a decrease in L-serine deaminase activity. J Bacteriol. 1985 Jan;161(1):272–276. doi: 10.1128/jb.161.1.272-276.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- PARDEE A. B., PRESTIDGE L. S. Induced formation of serine and threonine deaminases by Escherichia coli. J Bacteriol. 1955 Dec;70(6):667–674. doi: 10.1128/jb.70.6.667-674.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platko J. V., Willins D. A., Calvo J. M. The ilvIH operon of Escherichia coli is positively regulated. J Bacteriol. 1990 Aug;172(8):4563–4570. doi: 10.1128/jb.172.8.4563-4570.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quay S. C., Oxender D. L. Regulation of branched-chain amino acid transport in Escherichia coli. J Bacteriol. 1976 Sep;127(3):1225–1238. doi: 10.1128/jb.127.3.1225-1238.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raibaud O. Nucleoprotein structures at positively regulated bacterial promoters: homology with replication origins and some hypotheses on the quaternary structure of the activator proteins in these complexes. Mol Microbiol. 1989 Mar;3(3):455–458. doi: 10.1111/j.1365-2958.1989.tb00191.x. [DOI] [PubMed] [Google Scholar]
- Raibaud O., Vidal-Ingigliardi D., Richet E. A complex nucleoprotein structure involved in activation of transcription of two divergent Escherichia coli promoters. J Mol Biol. 1989 Feb 5;205(3):471–485. doi: 10.1016/0022-2836(89)90218-0. [DOI] [PubMed] [Google Scholar]
- Ricca E., Aker D. A., Calvo J. M. A protein that binds to the regulatory region of the Escherichia coli ilvIH operon. J Bacteriol. 1989 Mar;171(3):1658–1664. doi: 10.1128/jb.171.3.1658-1664.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H. S., Lang B. F., Newman E. B. L-serine degradation in Escherichia coli K-12: cloning and sequencing of the sdaA gene. J Bacteriol. 1989 Sep;171(9):5095–5102. doi: 10.1128/jb.171.9.5095-5102.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuan L. R., D'Ari R., Newman E. B. The leucine regulon of Escherichia coli K-12: a mutation in rblA alters expression of L-leucine-dependent metabolic operons. J Bacteriol. 1990 Aug;172(8):4529–4535. doi: 10.1128/jb.172.8.4529-4535.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincenz C., Fronk J., Tank G. A., Langmore J. P. Nucleoprotein hybridization: a method for isolating active and inactive genes as chromatin. Nucleic Acids Res. 1991 Mar 25;19(6):1325–1336. doi: 10.1093/nar/19.6.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willins D. A., Ryan C. W., Platko J. V., Calvo J. M. Characterization of Lrp, and Escherichia coli regulatory protein that mediates a global response to leucine. J Biol Chem. 1991 Jun 15;266(17):10768–10774. [PubMed] [Google Scholar]