Abstract
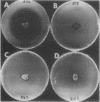
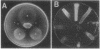
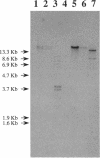
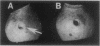
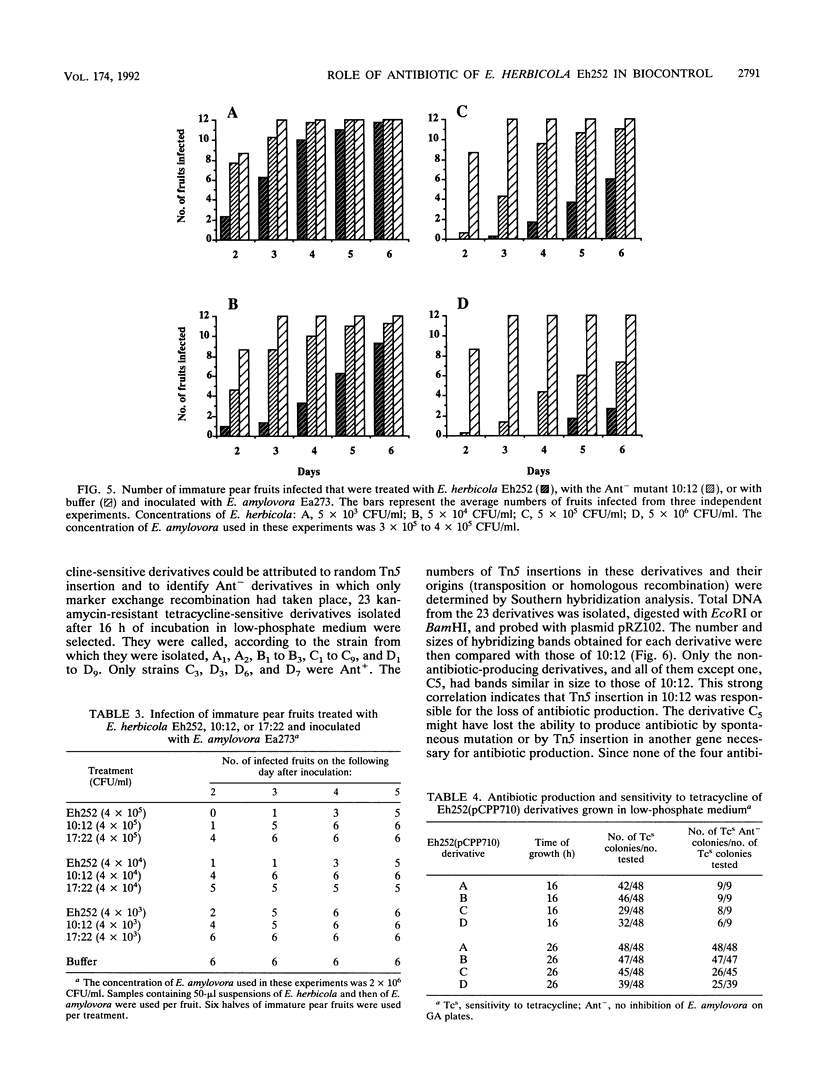
Erwinia herbicola Eh252 is a nonpathogenic epiphytic bacterium that reduces fire blight incidence when sprayed onto apple blossoms before inoculation with Erwinia amylovora, the causal agent of fire blight. Eh252 was found to produce on minimal medium an antibiotic that inhibited the growth of E. amylovora. This antibiotic was inactivated by histidine but not by Fe(II), was sensitive to proteolytic enzymes, and showed a narrow host range of activity. To determine the role of this antibiotic in the control of fire blight, two prototrophic Tn5-induced mutants, 10:12 and 17:12, that had lost their ability to inhibit E. amylovora on plates (Ant- mutants) were compared with the wild-type strain for their ability to suppress fire blight in immature pear fruits. The two mutants had single Tn5 insertions in the chromosome; although they grew in immature pear fruits at a rate similar to that of the wild-type strain, neither of these mutants suppressed fire blight as well as Eh252 did. The Tn5-containing fragment isolated from 10:12 was used to mutagenize Eh252 by marker exchange. Derivatives that acquired the Tn5-containing fragment by homologous recombination lost the ability to inhibit E. amylovora on minimal medium. Furthermore, the three Ant- derivatives tested were also affected in their ability to inhibit E. amylovora in immature pear fruits. The results obtained suggest that antibiotic production is a determinant of the biological control of E. amylovora by Eh252, but that another mechanism(s) is involved.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg D. E., Davies J., Allet B., Rochaix J. D. Transposition of R factor genes to bacteriophage lambda. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3628–3632. doi: 10.1073/pnas.72.9.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. K. Acceptance by Erwinia spp. of R plasmid R68.45 and its ability to mobilize the chromosome of Erwinia chrysanthemi. J Bacteriol. 1980 Apr;142(1):111–119. doi: 10.1128/jb.142.1.111-119.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. K., Gibbins L. N., Carpenter J. A. Some observations on the physiology of Erwinia herbicola and its possible implication as a factor antagonistic to Erwinia amylovora in the "fire-blight" syndrome. Can J Microbiol. 1969 Jun;15(6):640–642. [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erskine J. M., Lopatecki L. E. In vitro and in vivo interactions between Erwinia amylovora and related saprophytic bacteria. Can J Microbiol. 1975 Jan;21(1):35–41. doi: 10.1139/m75-005. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODMAN R. N. IN VITRO AND IN VIVO INTERACTIONS BETWEEN COMPONENTS OF MIXED BACTERIAL CULTURES ISOLATED FROM APPLE BUDS. Phytopathology. 1965 Feb;55:217–221. [PubMed] [Google Scholar]
- Jorgensen R. A., Rothstein S. J., Reznikoff W. S. A restriction enzyme cleavage map of Tn5 and location of a region encoding neomycin resistance. Mol Gen Genet. 1979;177(1):65–72. doi: 10.1007/BF00267254. [DOI] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz L. C., Zimm B. H. Size of DNA determined by viscoelastic measurements: results on bacteriophages, Bacillus subtilis and Escherichia coli. J Mol Biol. 1972 Dec 30;72(3):779–800. doi: 10.1016/0022-2836(72)90191-x. [DOI] [PubMed] [Google Scholar]
- Laviña M., Gaggero C., Moreno F. Microcin H47, a chromosome-encoded microcin antibiotic of Escherichia coli. J Bacteriol. 1990 Nov;172(11):6585–6588. doi: 10.1128/jb.172.11.6585-6588.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig R. A. Gene tandem-mediated selection of coliphage lambda-receptive Agrobacterium, Pseudomonas, and Rhizobium strains. Proc Natl Acad Sci U S A. 1987 May;84(10):3334–3338. doi: 10.1073/pnas.84.10.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray N. E., Brammar W. J., Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977 Jan 7;150(1):53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- Roeder D. L., Collmer A. Marker-exchange mutagenesis of a pectate lyase isozyme gene in Erwinia chrysanthemi. J Bacteriol. 1985 Oct;164(1):51–56. doi: 10.1128/jb.164.1.51-56.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J. Iron-Binding Catechols and Virulence in Escherichia coli. Infect Immun. 1973 Mar;7(3):445–456. doi: 10.1128/iai.7.3.445-456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwyn B., Neilands J. B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987 Jan;160(1):47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Thomashow L. S., Weller D. M. Role of a phenazine antibiotic from Pseudomonas fluorescens in biological control of Gaeumannomyces graminis var. tritici. J Bacteriol. 1988 Aug;170(8):3499–3508. doi: 10.1128/jb.170.8.3499-3508.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste J. L., Paulin J. P., Expert D. Bacteriophage Mu as a genetic tool to study Erwinia amylovora pathogenicity and hypersensitive reaction on tobacco. J Bacteriol. 1990 Feb;172(2):932–941. doi: 10.1128/jb.172.2.932-941.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelmann G., Lupp R., Jung G. Herbicolins--New peptide antibiotics from Erwinia herbicola. J Antibiot (Tokyo) 1980 Apr;33(4):353–358. doi: 10.7164/antibiotics.33.353. [DOI] [PubMed] [Google Scholar]
- de Vries G. E., Raymond C. K., Ludwig R. A. Extension of bacteriophage lambda host range: selection, cloning, and characterization of a constitutive lambda receptor gene. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6080–6084. doi: 10.1073/pnas.81.19.6080. [DOI] [PMC free article] [PubMed] [Google Scholar]