Abstract
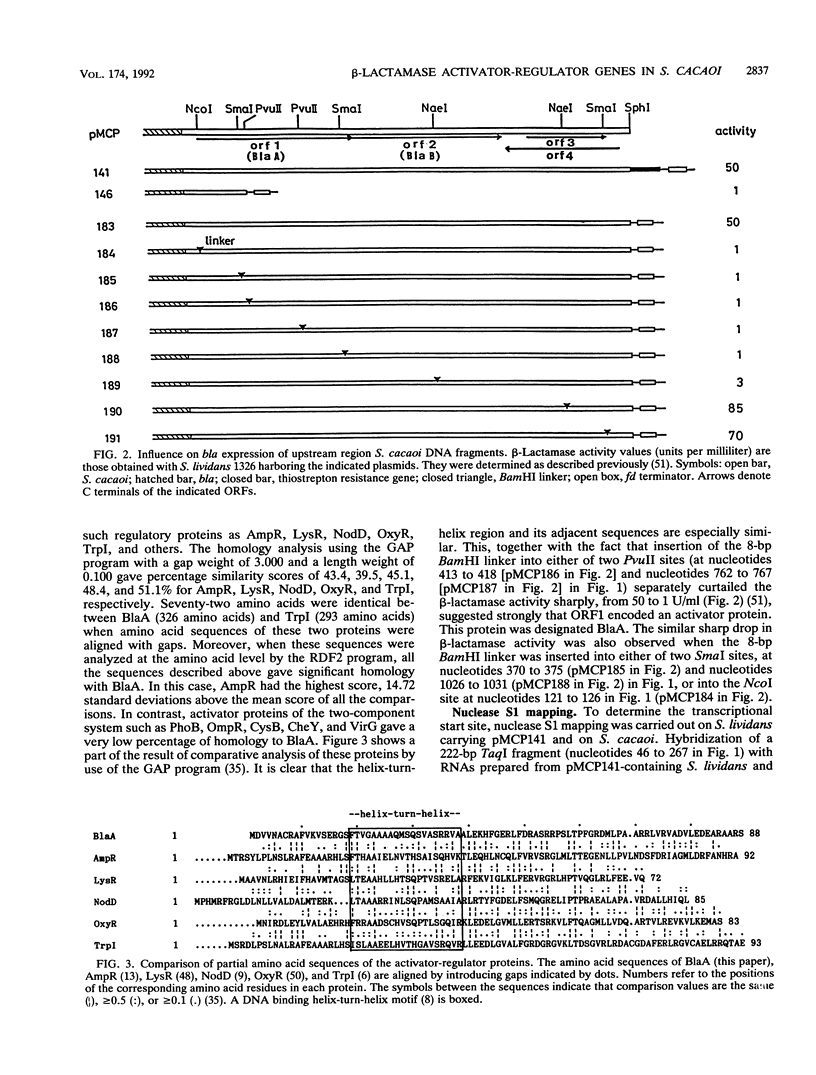
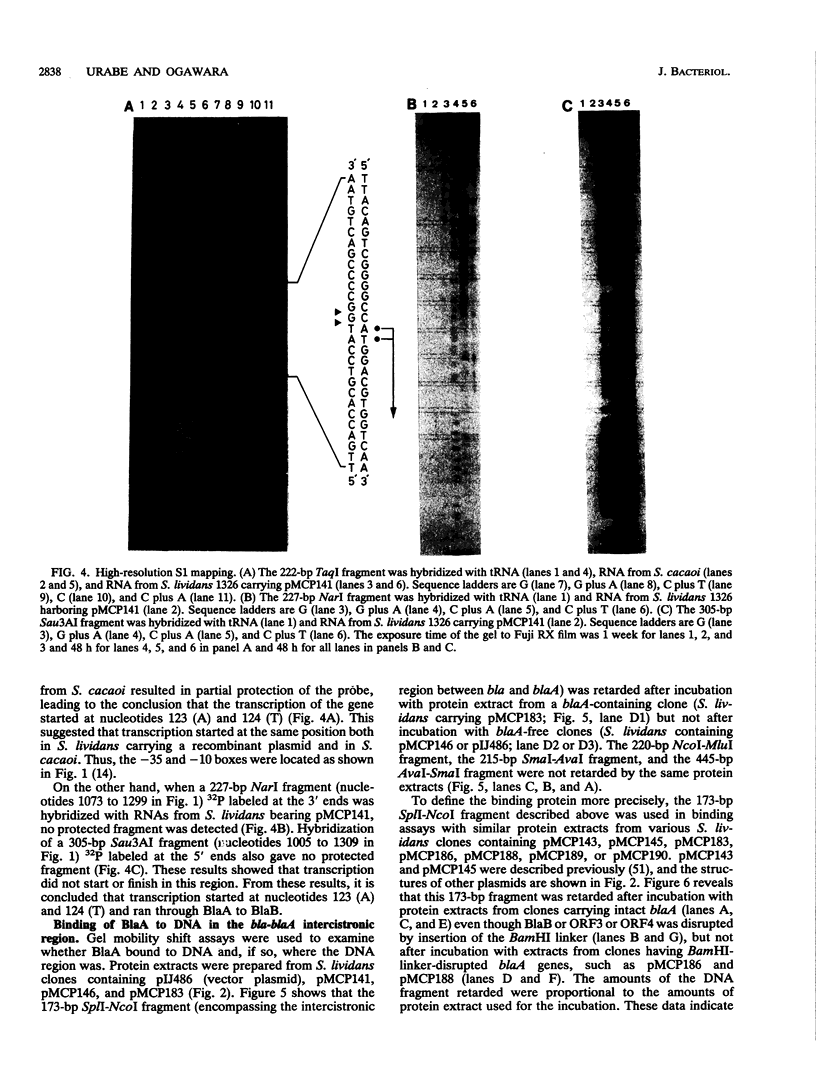
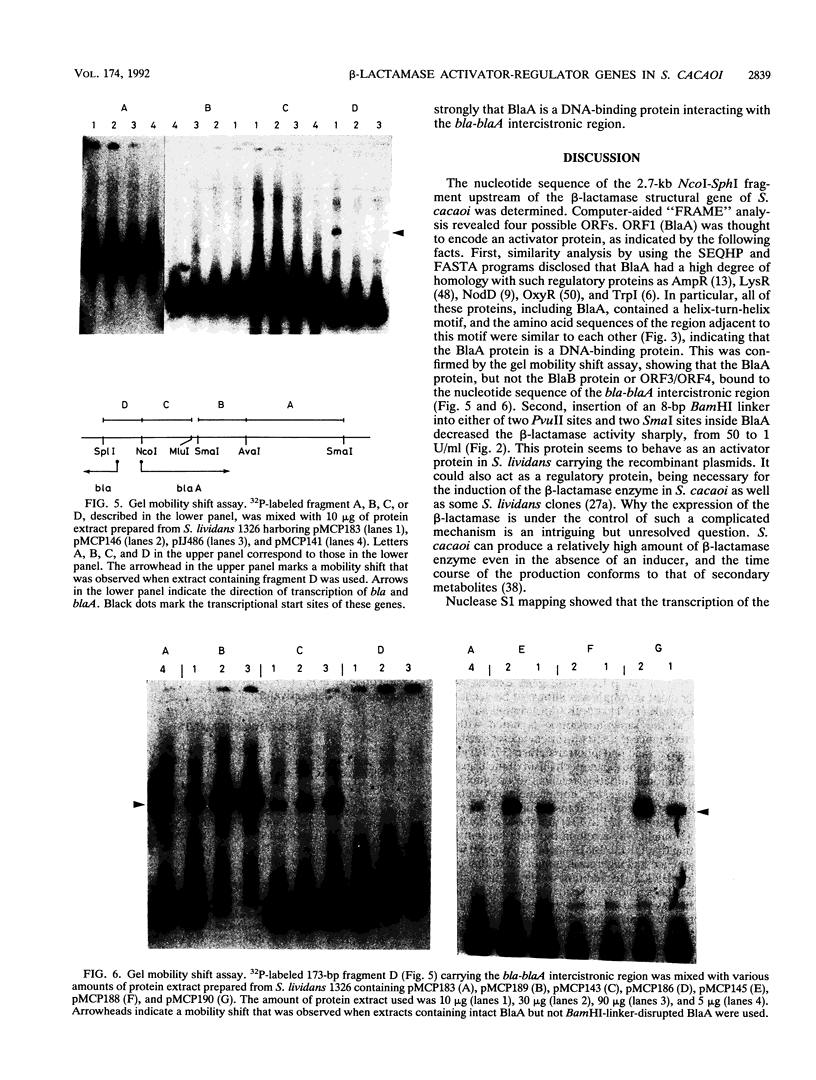
The nucleotide sequence of the 2.7-kb DNA fragment upstream of the structural gene of beta-lactamase in Streptomyces cacaoi was determined. Computer-aided "FRAME" analysis revealed four possible open reading frames (ORFs), three in one direction and one in the opposite direction. One of them (ORF1, BlaA) encoded an activator-regulator protein whose deduced amino acid sequence was similar to that of other activator-regulator proteins in bacteria. Insertion of an 8-bp BamHI linker into the BlaA region decreased the beta-lactamase activity sharply, from 50 U to 1 U/ml. This protein (BlaA) was found to bind to the nucleotide sequence between the bla (beta-lactamase structural gene) and blaA genes. Another ORF (ORF2, BlaB) in the same orientation had a couple of amino acid sequences similar to that of pBR322 beta-lactamase. However, insertion of the 8-bp BamHI linker indicated that this ORF was functional as an activator-regulator but not as a beta-lactamase. Therefore, there were two activator-regulator proteins in the upstream region of the structural gene of the beta-lactamase. Nuclease S1 mapping predicted that transcription for the activator proteins commenced at the translational initiation codon or within a few nucleotides from the translational start site. Transcription was in the opposite direction to that of the beta-lactamase structural gene.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartowsky E., Normark S. Purification and mutant analysis of Citrobacter freundii AmpR, the regulator for chromosomal AmpC beta-lactamase. Mol Microbiol. 1991 Jul;5(7):1715–1725. doi: 10.1111/j.1365-2958.1991.tb01920.x. [DOI] [PubMed] [Google Scholar]
- Baylis H. A., Bibb M. J. The nucleotide sequence of a 16S rRNA gene from Streptomyces coelicolor A3(2) Nucleic Acids Res. 1987 Sep 11;15(17):7176–7176. doi: 10.1093/nar/15.17.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb M. J., Findlay P. R., Johnson M. W. The relationship between base composition and codon usage in bacterial genes and its use for the simple and reliable identification of protein-coding sequences. Gene. 1984 Oct;30(1-3):157–166. doi: 10.1016/0378-1119(84)90116-1. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Janssen G. R., Ward J. M. Cloning and analysis of the promoter region of the erythromycin resistance gene (ermE) of Streptomyces erythraeus. Gene. 1985;38(1-3):215–226. doi: 10.1016/0378-1119(85)90220-3. [DOI] [PubMed] [Google Scholar]
- Chang M., Hadero A., Crawford I. P. Sequence of the Pseudomonas aeruginosa trpI activator gene and relatedness of trpI to other procaryotic regulatory genes. J Bacteriol. 1989 Jan;171(1):172–183. doi: 10.1128/jb.171.1.172-183.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd I. B., Egan J. B. Systematic method for the detection of potential lambda Cro-like DNA-binding regions in proteins. J Mol Biol. 1987 Apr 5;194(3):557–564. doi: 10.1016/0022-2836(87)90681-4. [DOI] [PubMed] [Google Scholar]
- Egelhoff T. T., Fisher R. F., Jacobs T. W., Mulligan J. T., Long S. R. Nucleotide sequence of Rhizobium meliloti 1021 nodulation genes: nodD is read divergently from nodABC. DNA. 1985 Jun;4(3):241–248. doi: 10.1089/dna.1985.4.241. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Honoré N., Nicolas M. H., Cole S. T. Inducible cephalosporinase production in clinical isolates of Enterobacter cloacae is controlled by a regulatory gene that has been deleted from Escherichia coli. EMBO J. 1986 Dec 20;5(13):3709–3714. doi: 10.1002/j.1460-2075.1986.tb04704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Furuya K., Nishiyama M., Suzuki H., Beppu T. Nucleotide sequence of the streptothricin acetyltransferase gene from Streptomyces lavendulae and its expression in heterologous hosts. J Bacteriol. 1987 May;169(5):1929–1937. doi: 10.1128/jb.169.5.1929-1937.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Kito M., Nishiyama M., Furuya K., Hong S. K., Miyake K., Beppu T. Primary structure of AfsR, a global regulatory protein for secondary metabolite formation in Streptomyces coelicolor A3(2). Gene. 1990 Oct 30;95(1):49–56. doi: 10.1016/0378-1119(90)90412-k. [DOI] [PubMed] [Google Scholar]
- Hoshiko S., Nojiri C., Matsunaga K., Katsumata K., Satoh E., Nagaoka K. Nucleotide sequence of the ribostamycin phosphotransferase gene and of its control region in Streptomyces ribosidificus. Gene. 1988 Sep 7;68(2):285–296. doi: 10.1016/0378-1119(88)90031-5. [DOI] [PubMed] [Google Scholar]
- Imanaka T., Himeno T., Aiba S. Cloning and nucleotide sequence of the penicillinase antirepressor gene penJ of Bacillus licheniformis. J Bacteriol. 1987 Sep;169(9):3867–3872. doi: 10.1128/jb.169.9.3867-3872.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen G. R., Ward J. M., Bibb M. J. Unusual transcriptional and translational features of the aminoglycoside phosphotransferase gene (aph) from Streptomyces fradiae. Genes Dev. 1989 Mar;3(3):415–429. doi: 10.1101/gad.3.3.415. [DOI] [PubMed] [Google Scholar]
- Jaurin B., Grundström T., Edlund T., Normark S. The E. coli beta-lactamase attenuator mediates growth rate-dependent regulation. Nature. 1981 Mar 19;290(5803):221–225. doi: 10.1038/290221a0. [DOI] [PubMed] [Google Scholar]
- Joris B., Ghuysen J. M., Dive G., Renard A., Dideberg O., Charlier P., Frère J. M., Kelly J. A., Boyington J. C., Moews P. C. The active-site-serine penicillin-recognizing enzymes as members of the Streptomyces R61 DD-peptidase family. Biochem J. 1988 Mar 1;250(2):313–324. doi: 10.1042/bj2500313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris B., Ledent P., Kobayashi T., Lampen J. O., Ghuysen J. M. Expression in Escherichia coli of the carboxy terminal domain of the BLAR sensory-transducer protein of Bacillus licheniformis as a water-soluble Mr 26,000 penicillin-binding protein. FEMS Microbiol Lett. 1990 Jun 15;58(1):107–113. doi: 10.1016/0378-1097(90)90111-3. [DOI] [PubMed] [Google Scholar]
- Kanehisa M. I. Los Alamos sequence analysis package for nucleic acids and proteins. Nucleic Acids Res. 1982 Jan 11;10(1):183–196. doi: 10.1093/nar/10.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieser T. Factors affecting the isolation of CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid. 1984 Jul;12(1):19–36. doi: 10.1016/0147-619x(84)90063-5. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Zhu Y. F., Nicholls N. J., Lampen J. O. A second regulatory gene, blaR1, encoding a potential penicillin-binding protein required for induction of beta-lactamase in Bacillus licheniformis. J Bacteriol. 1987 Sep;169(9):3873–3878. doi: 10.1128/jb.169.9.3873-3878.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzini M. V., Nojima S., Dusart J., Ogawara H., Dehottay P., Frere J. M., Ghuysen J. M. Cloning and amplified expression in Streptomyces lividans of the gene encoding the extracellular beta-lactamase from Streptomyces cacaoi. J Gen Microbiol. 1987 Oct;133(10):2915–2920. doi: 10.1099/00221287-133-10-2915. [DOI] [PubMed] [Google Scholar]
- Lindquist S., Lindberg F., Normark S. Binding of the Citrobacter freundii AmpR regulator to a single DNA site provides both autoregulation and activation of the inducible ampC beta-lactamase gene. J Bacteriol. 1989 Jul;171(7):3746–3753. doi: 10.1128/jb.171.7.3746-3753.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomovskaya N. D., Mkrtumian N. M., Gostimskaya N. L., Danilenko V. N. Characterization of temperate actinophage phi C31 isolated from Streptomyces coelicolor A3(2). J Virol. 1972 Feb;9(2):258–262. doi: 10.1128/jvi.9.2.258-262.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki M., Takano E., Mori H., Sato A., Murachi T., Hatanaka M. All four internally repetitive domains of pig calpastatin possess inhibitory activities against calpains I and II. FEBS Lett. 1987 Oct 19;223(1):174–180. doi: 10.1016/0014-5793(87)80531-8. [DOI] [PubMed] [Google Scholar]
- Matsuhashi Y., Murakami T., Nojiri C., Toyama H., Anzai H., Nagaoka K. Mechanisms of aminoglycoside-resistance of Streptomyces harboring resistant genes obtained from antibiotic-producers. J Antibiot (Tokyo) 1985 Feb;38(2):279–282. doi: 10.7164/antibiotics.38.279. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nakano M. M., Mashiko H., Ogawara H. Cloning of the kanamycin resistance gene from a kanamycin-producing Streptomyces species. J Bacteriol. 1984 Jan;157(1):79–83. doi: 10.1128/jb.157.1.79-83.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Ogawara H. Antibiotic resistance in pathogenic and producing bacteria, with special reference to beta-lactam antibiotics. Microbiol Rev. 1981 Dec;45(4):591–619. doi: 10.1128/mr.45.4.591-619.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawara H., Mantoku A., Shimada S. beta-lactamase from Streptomyces cacaoi. Purification and properties. J Biol Chem. 1981 Mar 25;256(6):2649–2655. [PubMed] [Google Scholar]
- Ogawara H. Production and property of beta-lactamases in Streptomyces. Antimicrob Agents Chemother. 1975 Oct;8(4):402–408. doi: 10.1128/aac.8.4.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura S., Ikeda H., Tanaka H. Extraction and characterization of plasmids from macrolide antibiotic-producing streptomycetes. J Antibiot (Tokyo) 1981 Apr;34(4):478–482. doi: 10.7164/antibiotics.34.478. [DOI] [PubMed] [Google Scholar]
- Ounissi H., Courvalin P. Nucleotide sequence of the gene ereA encoding the erythromycin esterase in Escherichia coli. Gene. 1985;35(3):271–278. doi: 10.1016/0378-1119(85)90005-8. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn M. D., Thireos G., Greer H. Temporal analysis of general control of amino acid biosynthesis in Saccharomyces cerevisiae: role of positive regulatory genes in initiation and maintenance of mRNA derepression. Mol Cell Biol. 1984 Mar;4(3):520–528. doi: 10.1128/mcb.4.3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen G. B., Stockwell P. A., Hill D. F. Messenger RNA recognition in Escherichia coli: a possible second site of interaction with 16S ribosomal RNA. EMBO J. 1988 Dec 1;7(12):3957–3962. doi: 10.1002/j.1460-2075.1988.tb03282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeggs P. A., Thompson J., Cundliffe E. Methylation of 16S ribosomal RNA and resistance to aminoglycoside antibiotics in clones of Streptomyces lividans carrying DNA from Streptomyces tenjimariensis. Mol Gen Genet. 1985;200(3):415–421. doi: 10.1007/BF00425725. [DOI] [PubMed] [Google Scholar]
- Smith H. O. Recovery of DNA from gels. Methods Enzymol. 1980;65(1):371–380. doi: 10.1016/s0076-6879(80)65048-4. [DOI] [PubMed] [Google Scholar]
- Stragier P., Patte J. C. Regulation of diaminopimelate decarboxylase synthesis in Escherichia coli. III. Nucleotide sequence and regulation of the lysR gene. J Mol Biol. 1983 Aug 5;168(2):333–350. doi: 10.1016/s0022-2836(83)80022-9. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. Nucleotide sequence of the ampicillin resistance gene of Escherichia coli plasmid pBR322. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3737–3741. doi: 10.1073/pnas.75.8.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao K., Makino K., Yonei S., Nakata A., Shinagawa H. Molecular cloning and nucleotide sequencing of oxyR, the positive regulatory gene of a regulon for an adaptive response to oxidative stress in Escherichia coli: homologies between OxyR protein and a family of bacterial activator proteins. Mol Gen Genet. 1989 Sep;218(3):371–376. doi: 10.1007/BF00332397. [DOI] [PubMed] [Google Scholar]
- Urabe H., Lenzini M. V., Mukaide M., Dusart J., Nakano M. M., Ghuysen J. M., Ogawara H. Beta-lactamase expression in Streptomyces cacaoi. J Bacteriol. 1990 Nov;172(11):6427–6434. doi: 10.1128/jb.172.11.6427-6434.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Ward J. M., Janssen G. R., Kieser T., Bibb M. J., Buttner M. J., Bibb M. J. Construction and characterisation of a series of multi-copy promoter-probe plasmid vectors for Streptomyces using the aminoglycoside phosphotransferase gene from Tn5 as indicator. Mol Gen Genet. 1986 Jun;203(3):468–478. doi: 10.1007/BF00422072. [DOI] [PubMed] [Google Scholar]