Abstract
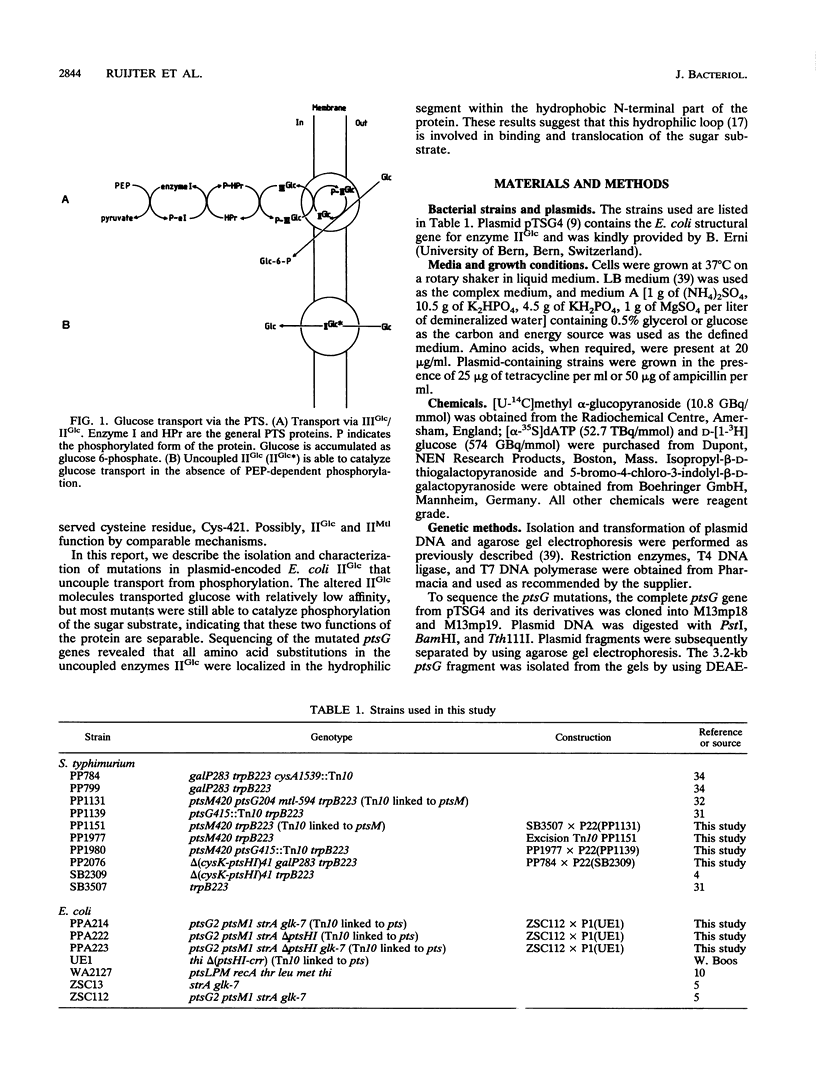
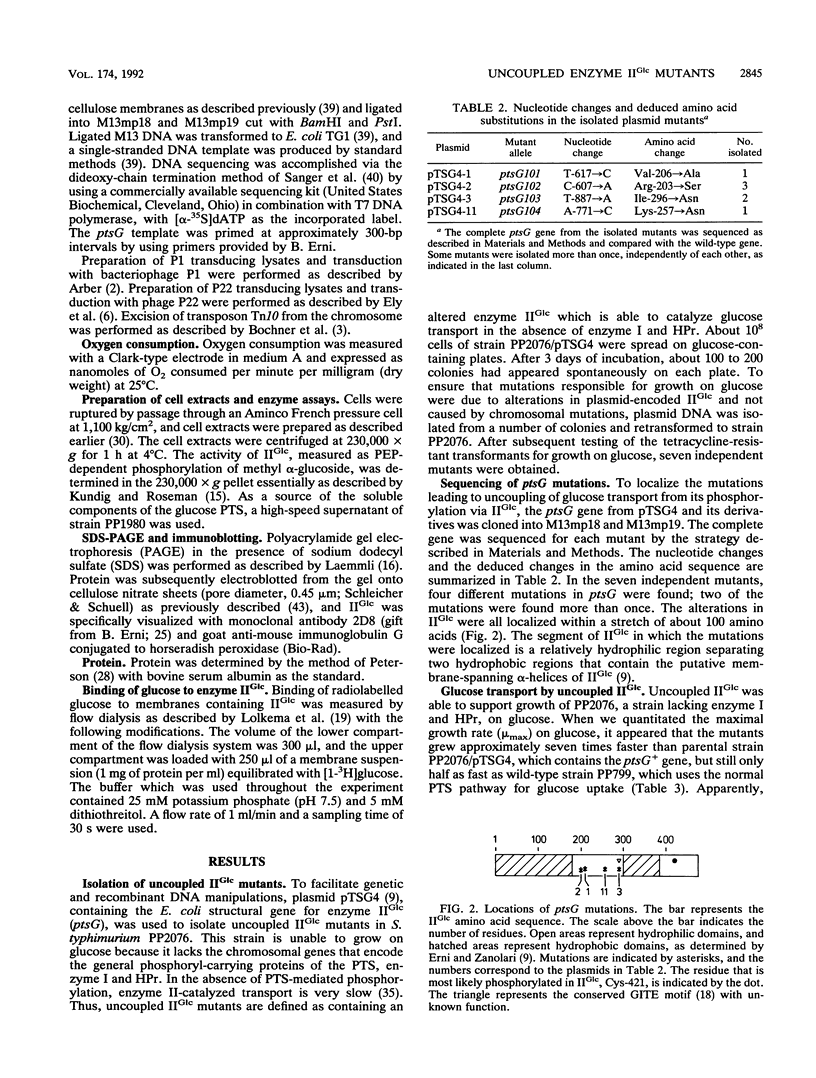
Mutations that uncouple glucose transport from phosphorylation were isolated in plasmid-encoded Escherichia coli enzyme IIGlc of the phosphoenolpyruvate-dependent phosphotransferase system (PTS). The uncoupled enzymes IIGlc were able to transport glucose in the absence of the general phosphoryl-carrying proteins of the PTS, enzyme I and HPr, although with relatively low affinity. Km values of the uncoupled enzymes IIGlc for glucose ranged from 0.5 to 2.5 mM, 2 orders of magnitude higher than the value of normal IIGlc. Most of the mutant proteins were still able to phosphorylate glucose and methyl alpha-glucoside (a non-metabolizable glucose analog specific for IIGlc), indicating that transport and phosphorylation are separable functions of the enzyme. Some of the uncoupled enzymes IIGlc transported glucose with a higher rate and lower apparent Km in a pts+ strain than in a delta ptsHI strain lacking the general proteins enzyme I and HPr. Since the properties of these uncoupled enzymes IIGlc in the presence of PTS-mediated phosphoryl transfer resembled those of wild-type IIGlc, these mutants appeared to be conditionally uncoupled. Sequencing of the mutated ptsG genes revealed that all amino acid substitutions occurred in a hydrophilic segment within the hydrophobic N-terminal part of IIGlc. These results suggest that this hydrophilic loop is involved in binding and translocation of the sugar substrate.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARBER W. Transduction of chromosomal genes and episomes in Escherichia coli. Virology. 1960 May;11:273–288. doi: 10.1016/0042-6822(60)90066-0. [DOI] [PubMed] [Google Scholar]
- Adler J., Epstein W. Phosphotransferase-system enzymes as chemoreceptors for certain sugars in Escherichia coli chemotaxis. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2895–2899. doi: 10.1073/pnas.71.7.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner B. R., Huang H. C., Schieven G. L., Ames B. N. Positive selection for loss of tetracycline resistance. J Bacteriol. 1980 Aug;143(2):926–933. doi: 10.1128/jb.143.2.926-933.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordaro J. C., Roseman S. Deletion mapping of the genes coding for HPr and enzyme I of the phosphoenolpyruvate: sugar phosphotransferase system in Salmonella typhimurium. J Bacteriol. 1972 Oct;112(1):17–29. doi: 10.1128/jb.112.1.17-29.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis S. J., Epstein W. Phosphorylation of D-glucose in Escherichia coli mutants defective in glucosephosphotransferase, mannosephosphotransferase, and glucokinase. J Bacteriol. 1975 Jun;122(3):1189–1199. doi: 10.1128/jb.122.3.1189-1199.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erni B. Glucose transport in Escherichia coli. FEMS Microbiol Rev. 1989 Jun;5(1-2):13–23. doi: 10.1016/0168-6445(89)90004-1. [DOI] [PubMed] [Google Scholar]
- Erni B. Glucose-specific permease of the bacterial phosphotransferase system: phosphorylation and oligomeric structure of the glucose-specific IIGlc-IIIGlc complex of Salmonella typhimurium. Biochemistry. 1986 Jan 28;25(2):305–312. doi: 10.1021/bi00350a004. [DOI] [PubMed] [Google Scholar]
- Erni B., Zanolari B. Glucose-permease of the bacterial phosphotransferase system. Gene cloning, overproduction, and amino acid sequence of enzyme IIGlc. J Biol Chem. 1986 Dec 15;261(35):16398–16403. [PubMed] [Google Scholar]
- Erni B., Zanolari B., Graff P., Kocher H. P. Mannose permease of Escherichia coli. Domain structure and function of the phosphorylating subunit. J Biol Chem. 1989 Nov 5;264(31):18733–18741. [PubMed] [Google Scholar]
- Grisafi P. L., Scholle A., Sugiyama J., Briggs C., Jacobson G. R., Lengeler J. W. Deletion mutants of the Escherichia coli K-12 mannitol permease: dissection of transport-phosphorylation, phospho-exchange, and mannitol-binding activities. J Bacteriol. 1989 May;171(5):2719–2727. doi: 10.1128/jb.171.5.2719-2727.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman P. E. Some improved methods in P22 transduction. Genetics. 1974 Apr;76(4):625–631. doi: 10.1093/genetics/76.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S. C., Wilson T. H. Characterization of Escherichia coli lactose carrier mutants that transport protons without a cosubstrate. Probes for the energy barrier to uncoupled transport. J Biol Chem. 1990 Jun 15;265(17):9645–9651. [PubMed] [Google Scholar]
- King S. C., Wilson T. H. Identification of valine 177 as a mutation altering specificity for transport of sugars by the Escherichia coli lactose carrier. Enhanced specificity for sucrose and maltose. J Biol Chem. 1990 Jun 15;265(17):9638–9644. [PubMed] [Google Scholar]
- Kornberg H. L., Riordan C. Uptake of galactose into Escherichia coli by facilitated diffusion. J Gen Microbiol. 1976 May;94(1):75–89. doi: 10.1099/00221287-94-1-75. [DOI] [PubMed] [Google Scholar]
- Kundig W., Roseman S. Sugar transport. II. Characterization of constitutive membrane-bound enzymes II of the Escherichia coli phosphotransferase system. J Biol Chem. 1971 Mar 10;246(5):1407–1418. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lengeler J. W., Titgemeyer F., Vogler A. P., Wöhrl B. M. Structures and homologies of carbohydrate: phosphotransferase system (PTS) proteins. Philos Trans R Soc Lond B Biol Sci. 1990 Jan 30;326(1236):489–504. doi: 10.1098/rstb.1990.0027. [DOI] [PubMed] [Google Scholar]
- Lolkema J. S., Dijkstra D. S., ten Hoeve-Duurkens R. H., Robillard G. T. Interaction between the cytoplasmic and membrane-bound domains of enzyme IImtl of the Escherichia coli phosphoenolpyruvate-dependent phosphotransferase system. Biochemistry. 1991 Jul 9;30(27):6721–6726. doi: 10.1021/bi00241a013. [DOI] [PubMed] [Google Scholar]
- Lolkema J. S., Dijkstra D. S., ten Hoeve-Duurkens R. H., Robillard G. T. The membrane-bound domain of the phosphotransferase enzyme IImtl of Escherichia coli constitutes a mannitol translocating unit. Biochemistry. 1990 Nov 27;29(47):10659–10663. doi: 10.1021/bi00499a012. [DOI] [PubMed] [Google Scholar]
- Lolkema J. S., ten Hoeve-Duurkens R. H., Dijkstra D. S., Robillard G. T. Mechanistic coupling of transport and phosphorylation activity by enzyme IImtl of the Escherichia coli phosphoenolpyruvate-dependent phosphotransferase system. Biochemistry. 1991 Jul 9;30(27):6716–6721. doi: 10.1021/bi00241a012. [DOI] [PubMed] [Google Scholar]
- Manayan R., Tenn G., Yee H. B., Desai J. D., Yamada M., Saier M. H., Jr Genetic analyses of the mannitol permease of Escherichia coli: isolation and characterization of a transport-deficient mutant which retains phosphorylation activity. J Bacteriol. 1988 Mar;170(3):1290–1296. doi: 10.1128/jb.170.3.1290-1296.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall-Carlson L., Celenza J. L., Laurent B. C., Carlson M. Mutational analysis of the SNF3 glucose transporter of Saccharomyces cerevisiae. Mol Cell Biol. 1990 Mar;10(3):1105–1115. doi: 10.1128/mcb.10.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadow N. D., Fox D. K., Roseman S. The bacterial phosphoenolpyruvate: glycose phosphotransferase system. Annu Rev Biochem. 1990;59:497–542. doi: 10.1146/annurev.bi.59.070190.002433. [DOI] [PubMed] [Google Scholar]
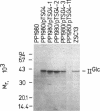
- Meins M., Zanolari B., Rosenbusch J. P., Erni B. Glucose permease of Escherichia coli. Purification of the IIGlc subunit and functional characterization of its oligomeric forms. J Biol Chem. 1988 Sep 15;263(26):12986–12993. [PubMed] [Google Scholar]
- Nuoffer C., Zanolari B., Erni B. Glucose permease of Escherichia coli. The effect of cysteine to serine mutations on the function, stability, and regulation of transport and phosphorylation. J Biol Chem. 1988 May 15;263(14):6647–6655. [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Postma P. W. Defective enzyme II-BGlc of the phosphoenolpyruvate:sugar phosphotransferase system leading to uncoupling of transport and phosphorylation in Salmonella typhimurium. J Bacteriol. 1981 Aug;147(2):382–389. doi: 10.1128/jb.147.2.382-389.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W. Galactose transport in Salmonella typhimurium. J Bacteriol. 1977 Feb;129(2):630–639. doi: 10.1128/jb.129.2.630-639.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W. Involvement of the phosphotransferase system in galactose transport in Salmonella typhimurium. FEBS Lett. 1976 Jan 1;61(1):49–53. doi: 10.1016/0014-5793(76)80169-x. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Keizer H. G., Koolwijk P. Transport of trehalose in Salmonella typhimurium. J Bacteriol. 1986 Dec;168(3):1107–1111. doi: 10.1128/jb.168.3.1107-1111.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Lengeler J. W. Phosphoenolpyruvate:carbohydrate phosphotransferase system of bacteria. Microbiol Rev. 1985 Sep;49(3):232–269. doi: 10.1128/mr.49.3.232-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Schuitema A., Kwa C. Regulation of methyl beta-galactoside permease activity in pts and crr mutants of Salmonella typhimurium. Mol Gen Genet. 1981;181(4):448–453. doi: 10.1007/BF00428734. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Stock J. B. Enzymes II of the phosphotransferase system do not catalyze sugar transport in the absence of phosphorylation. J Bacteriol. 1980 Feb;141(2):476–484. doi: 10.1128/jb.141.2.476-484.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robillard G. T., Lolkema J. S. Enzymes II of the phosphoenolpyruvate-dependent sugar transport systems: a review of their structure and mechanism of sugar transport. Biochim Biophys Acta. 1988 Oct 11;947(3):493–519. doi: 10.1016/0304-4157(88)90005-6. [DOI] [PubMed] [Google Scholar]
- Ruijter G. J., Postma P. W., van Dam K. Adaptation of Salmonella typhimurium mutants containing uncoupled enzyme IIGlc to glucose-limited conditions. J Bacteriol. 1990 Sep;172(9):4783–4789. doi: 10.1128/jb.172.9.4783-4789.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruijter G. J., Postma P. W., van Dam K. Energetics of glucose uptake in a Salmonella typhimurium mutant containing uncoupled enzyme IIGlc. Arch Microbiol. 1991;155(3):234–237. doi: 10.1007/BF00252206. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnetz K., Sutrina S. L., Saier M. H., Jr, Rak B. Identification of catalytic residues in the beta-glucoside permease of Escherichia coli by site-specific mutagenesis and demonstration of interdomain cross-reactivity between the beta-glucoside and glucose systems. J Biol Chem. 1990 Aug 15;265(23):13464–13471. [PubMed] [Google Scholar]
- Stephan M. M., Khandekar S. S., Jacobson G. R. Hydrophilic C-terminal domain of the Escherichia coli mannitol permease: phosphorylation, functional independence, and evidence for intersubunit phosphotransfer. Biochemistry. 1989 Sep 19;28(19):7941–7946. doi: 10.1021/bi00445a058. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. W., Jacobson G. R. Molecular cloning of the C-terminal domain of Escherichia coli D-mannitol permease: expression, phosphorylation, and complementation with C-terminal permease deletion proteins. J Bacteriol. 1990 Mar;172(3):1509–1515. doi: 10.1128/jb.172.3.1509-1515.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Weeghel R. P., Meyer G. H., Keck W., Robillard G. T. Phosphoenolpyruvate-dependent mannitol phosphotransferase system of Escherichia coli: overexpression, purification, and characterization of the enzymatically active C-terminal domain of enzyme IImtl equivalent to enzyme IIImtl. Biochemistry. 1991 Feb 19;30(7):1774–1779. doi: 10.1021/bi00221a007. [DOI] [PubMed] [Google Scholar]
- van Weeghel R. P., Meyer G., Pas H. H., Keck W., Robillard G. T. Cytoplasmic phosphorylating domain of the mannitol-specific transport protein of the phosphoenolpyruvate-dependent phosphotransferase system in Escherichia coli: overexpression, purification, and functional complementation with the mannitol binding domain. Biochemistry. 1991 Oct 1;30(39):9478–9485. doi: 10.1021/bi00103a013. [DOI] [PubMed] [Google Scholar]