Abstract
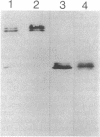
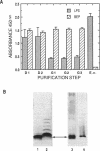
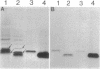
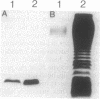
The ability of Bdellovibrio bacteriovorus to relocalize the OmpF major outer membrane porins from its Escherichia coli prey to its own outer membranes is diminished in prey expressing smooth lipopolysaccharide (S-LPS). Since porins exist in the membrane complexed with LPS, we examined the LPS associated with relocalized porin to determine whether it had been acquired intact, mixed or replaced with Bdellovibrio LPS, or derivatized by the bdellovibrios. The relocalized trimers were found associated with the same LPS originally bound to them in the E. coli. The bulk-phase LPS from bdellovibrios grown on various chemotypes of rough prey was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis to determine whether more than the trimer-bound LPS was acquired by the bdellovibrios. This analysis revealed bands of Bdellovibrio LPS matching the LPS chemotype of the prey. One or two other bands were identical in migration to the LPS of prey-independent mutants of B. bacteriovorus and represented bdellovibrio-synthesized LPS. The LPS of bdellovibrios grown on prey with radiolabeled lipid A showed radioactivity only in gel band positions identical with those of the prey's LPS. The amount of this prey-derived LPS was shown by enzyme-linked immunosorbent assay to reach a constant value during the purification of the bdellovibrios, and it represented approximately 25% of the total Bdellovibrio LPS. Immunoelectron microscopy confirmed the presence of prey-derived LPS on the cell surface of bdellovibrios, and no evidence could be found for bdellovibrio-induced modifications of the relocalized prey LPS.
Full text
PDF


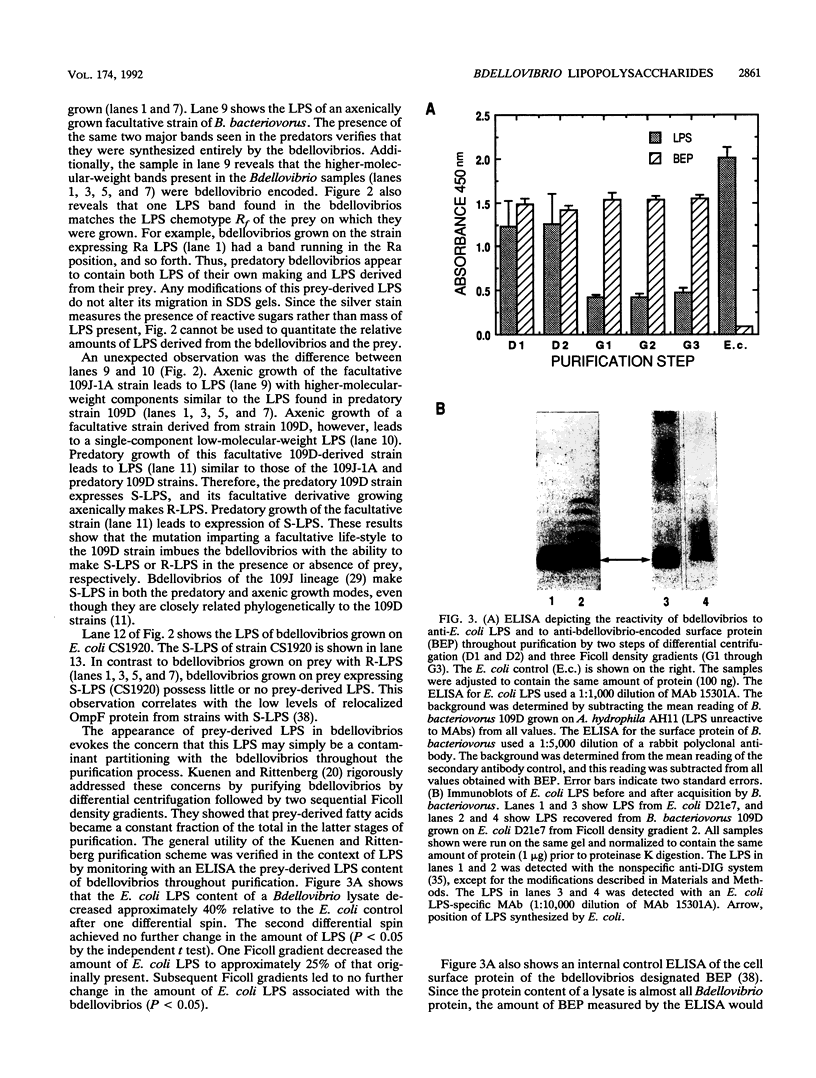

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abram D., Davis B. K. Structural properties and features of parasitic Bdellovibrio bacteriovorus. J Bacteriol. 1970 Nov;104(2):948–965. doi: 10.1128/jb.104.2.948-965.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman H. G., Monner D. A. Characterization of lipopolysaccharides from Escherichia coli K-12 mutants. J Bacteriol. 1975 Feb;121(2):455–464. doi: 10.1128/jb.121.2.455-464.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
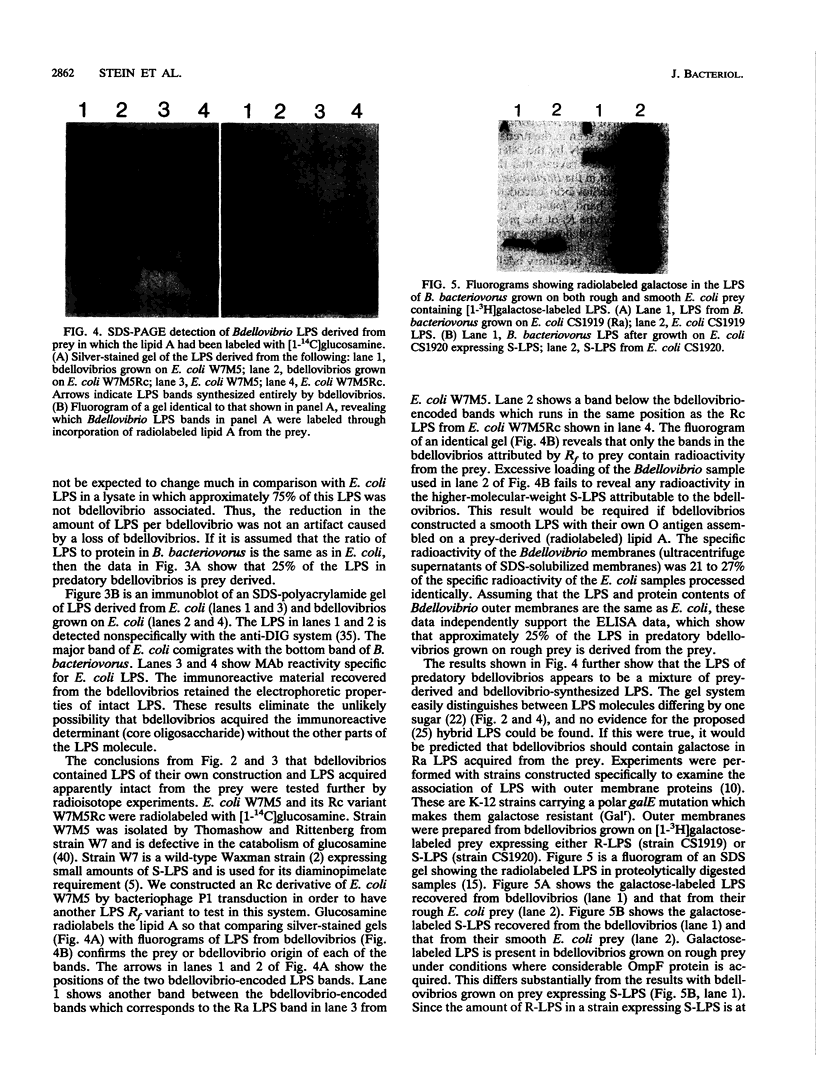
- DAVIS B. D. Biosynthetic interrelations of lysine, diaminopimelic acid, and threonine in mutants of Escherichia coli. Nature. 1952 Mar 29;169(4300):534–536. doi: 10.1038/169534a0. [DOI] [PubMed] [Google Scholar]
- Diano M., Le Bivic A., Hirn M. A method for the production of highly specific polyclonal antibodies. Anal Biochem. 1987 Oct;166(1):224–229. doi: 10.1016/0003-2697(87)90568-9. [DOI] [PubMed] [Google Scholar]
- Diedrich D. L. Bdellovibrios: recycling, remodelling and relocalizing components from their prey. Microbiol Sci. 1988 Apr;5(4):100–103. [PubMed] [Google Scholar]
- Diedrich D. L., Duran C. P., Conti S. F. Acquisition of Escherichia coli outer membrane proteins by Bdellovibrio sp. strain 109D. J Bacteriol. 1984 Jul;159(1):329–334. doi: 10.1128/jb.159.1.329-334.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrich D. L., Stein M. A., Schnaitman C. A. Associations of Escherichia coli K-12 OmpF trimers with rough and smooth lipopolysaccharides. J Bacteriol. 1990 Sep;172(9):5307–5311. doi: 10.1128/jb.172.9.5307-5311.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn S. D. Effects of the modification of transfer buffer composition and the renaturation of proteins in gels on the recognition of proteins on Western blots by monoclonal antibodies. Anal Biochem. 1986 Aug 15;157(1):144–153. doi: 10.1016/0003-2697(86)90207-1. [DOI] [PubMed] [Google Scholar]
- Flynn P. M., Shenep J. L., Gigliotti F., Davis D. S., Hildner W. K. Immunolabeling of lipopolysaccharide liberated from antibiotic-treated Escherichia coli. Infect Immun. 1988 Oct;56(10):2760–2762. doi: 10.1128/iai.56.10.2760-2762.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovin T. M. Multiphasic zone electrophoresis. I. Steady-state moving-boundary systems formed by different electrolyte combinations. Biochemistry. 1973 Feb 27;12(5):871–879. doi: 10.1021/bi00729a014. [DOI] [PubMed] [Google Scholar]
- Jovin T. M. Multiphasic zone electrophoresis. II. Design of integrated discontinuous buffer systems for analytical and preparative fractionation. Biochemistry. 1973 Feb 27;12(5):879–890. doi: 10.1021/bi00729a015. [DOI] [PubMed] [Google Scholar]
- Kuenen J. G., Rittenberg S. C. Incorporation of long-chain fatty acids of the substrate organism by Bdellovibrio bacteriovorus during intraperiplasmic growth. J Bacteriol. 1975 Mar;121(3):1145–1157. doi: 10.1128/jb.121.3.1145-1157.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
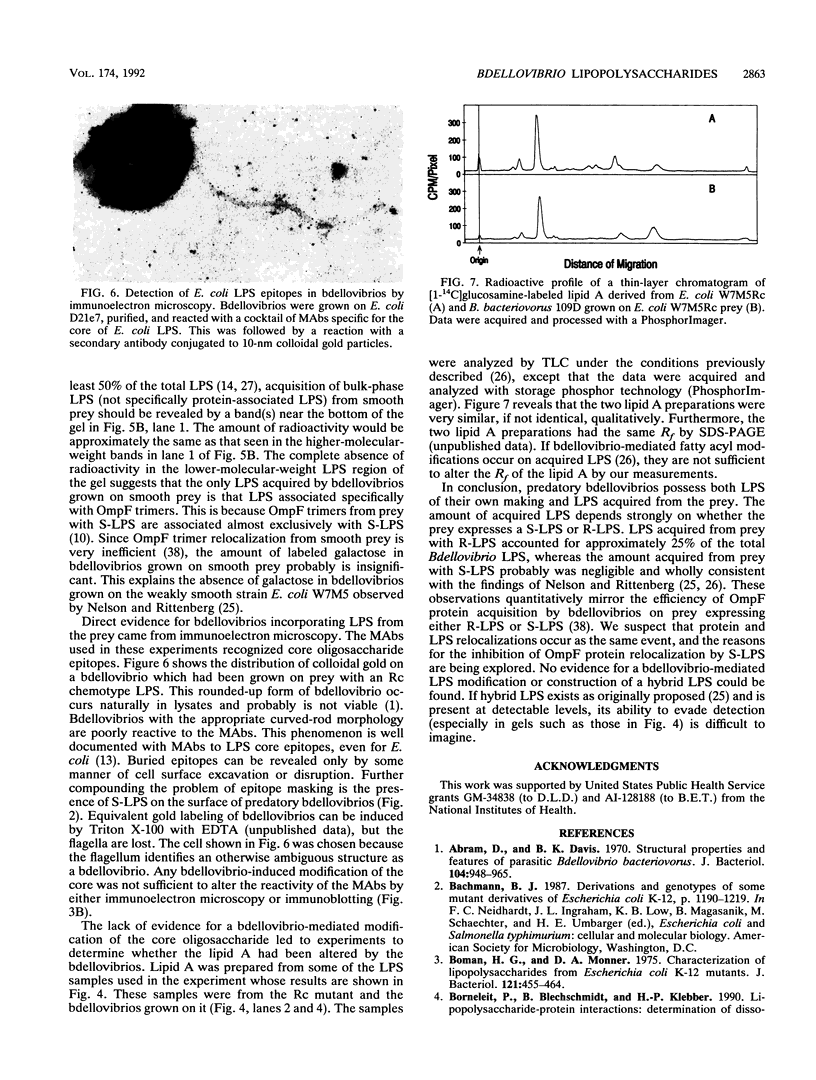
- Kulisek E. S., Holm S. E., Johnston K. H. A chromogenic assay for the detection of plasmin generated by plasminogen activator immobilized on nitrocellulose using a para-nitroanilide synthetic peptide substrate. Anal Biochem. 1989 Feb 15;177(1):78–84. doi: 10.1016/0003-2697(89)90017-1. [DOI] [PubMed] [Google Scholar]
- Lesse A. J., Campagnari A. A., Bittner W. E., Apicella M. A. Increased resolution of lipopolysaccharides and lipooligosaccharides utilizing tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Immunol Methods. 1990 Jan 24;126(1):109–117. doi: 10.1016/0022-1759(90)90018-q. [DOI] [PubMed] [Google Scholar]
- Lostrom M. E., Stone M. R., Tam M., Burnette W. N., Pinter A., Nowinski R. C. Monoclonal antibodies against murine leukemia viruses: identification of six antigenic determinants on the p 15(E) and gp70 envelope proteins. Virology. 1979 Oct 30;98(2):336–350. doi: 10.1016/0042-6822(79)90557-9. [DOI] [PubMed] [Google Scholar]
- Nelson D. R., Rittenberg S. C. Incorporation of substrate cell lipid A components into the lipopolysaccharide of intraperiplasmically grown Bdellovibrio bacteriovorus. J Bacteriol. 1981 Sep;147(3):860–868. doi: 10.1128/jb.147.3.860-868.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. R., Rittenberg S. C. Partial characterization of lipid A of intraperiplasmically grown Bdellovibrio bacteriovorus. J Bacteriol. 1981 Sep;147(3):869–874. doi: 10.1128/jb.147.3.869-874.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva E. T., Mäkelä P. H. Lipopolysaccharide heterogeneity in Salmonella typhimurium analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Eur J Biochem. 1980;107(1):137–143. doi: 10.1111/j.1432-1033.1980.tb04634.x. [DOI] [PubMed] [Google Scholar]
- Redinbaugh M. G., Turley R. B. Adaptation of the bicinchoninic acid protein assay for use with microtiter plates and sucrose gradient fractions. Anal Biochem. 1986 Mar;153(2):267–271. doi: 10.1016/0003-2697(86)90091-6. [DOI] [PubMed] [Google Scholar]
- Rittenberg S. C. Nonidentity of Bdellovibrio bacteriovorus strains 109D and 109J. J Bacteriol. 1972 Jan;109(1):432–433. doi: 10.1128/jb.109.1.432-433.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocque W. J., Coughlin R. T., McGroarty E. J. Lipopolysaccharide tightly bound to porin monomers and trimers from Escherichia coli K-12. J Bacteriol. 1987 Sep;169(9):4003–4010. doi: 10.1128/jb.169.9.4003-4010.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby E. G., McCabe J. B., Barke J. I. Uptake of intact nucleoside monophosphates by Bdellovibrio bacteriovorus 109J. J Bacteriol. 1985 Sep;163(3):1087–1094. doi: 10.1128/jb.163.3.1087-1094.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOLP H., STARR M. P. BDELLOVIBRIO BACTERIOVORUS GEN. ET SP. N., A PREDATORY, ECTOPARASITIC, AND BACTERIOLYTIC MICROORGANISM. Antonie Van Leeuwenhoek. 1963;29:217–248. doi: 10.1007/BF02046064. [DOI] [PubMed] [Google Scholar]
- Schindler H., Rosenbusch J. P. Matrix protein from Escherichia coli outer membranes forms voltage-controlled channels in lipid bilayers. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3751–3755. doi: 10.1073/pnas.75.8.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A., Austin E. A. Efficient incorporation of galactose into lipopolysaccharide by Escherichia coli K-12 strains with polar galE mutations. J Bacteriol. 1990 Sep;172(9):5511–5513. doi: 10.1128/jb.172.9.5511-5513.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A., McDonald G. A. Regulation of outer membrane protein synthesis in Escherichia coli K-12: deletion of ompC affects expression of the OmpF protein. J Bacteriol. 1984 Aug;159(2):555–563. doi: 10.1128/jb.159.2.555-563.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M. A., McAllister S. A., Johnston K. H., Diedrich D. L. Detection of lipopolysaccharides blotted to polyvinylidene difluoride membranes. Anal Biochem. 1990 Aug 1;188(2):285–287. doi: 10.1016/0003-2697(90)90607-b. [DOI] [PubMed] [Google Scholar]
- Sturm S., Timmis K. N. Cloning of the rfb gene region of Shigella dysenteriae 1 and construction of an rfb-rfp gene cassette for the development of lipopolysaccharide-based live anti-dysentery vaccines. Microb Pathog. 1986 Jun;1(3):289–297. doi: 10.1016/0882-4010(86)90054-9. [DOI] [PubMed] [Google Scholar]
- Talley B. G., McDade R. L., Jr, Diedrich D. L. Verification of the protein in the outer membrane of Bdellovibrio bacteriovorus as the OmpF protein of its Escherichia coli prey. J Bacteriol. 1987 Feb;169(2):694–698. doi: 10.1128/jb.169.2.694-698.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow M. F., Rittenberg S. C. Intraperiplasmic growth of Bdellovibrio bacteriovorus 109J: solubilization of Escherichia coli peptidoglycan. J Bacteriol. 1978 Sep;135(3):998–1007. doi: 10.1128/jb.135.3.998-1007.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torian B. E., Connelly R. J., Stephens R. S., Stibbs H. H. Specific and common antigens of Trichomonas vaginalis detected by monoclonal antibodies. Infect Immun. 1984 Jan;43(1):270–275. doi: 10.1128/iai.43.1.270-275.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torian B. E., Flores B. M., Stroeher V. L., Hagen F. S., Stamm W. E. cDNA sequence analysis of a 29-kDa cysteine-rich surface antigen of pathogenic Entamoeba histolytica. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6358–6362. doi: 10.1073/pnas.87.16.6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H., Mizushima S. Interaction between major outer membrane protein (O-8) and lipopolysaccharide in Escherichia coli K12. Eur J Biochem. 1980 Jan;103(1):209–218. doi: 10.1111/j.1432-1033.1980.tb04305.x. [DOI] [PubMed] [Google Scholar]