Abstract
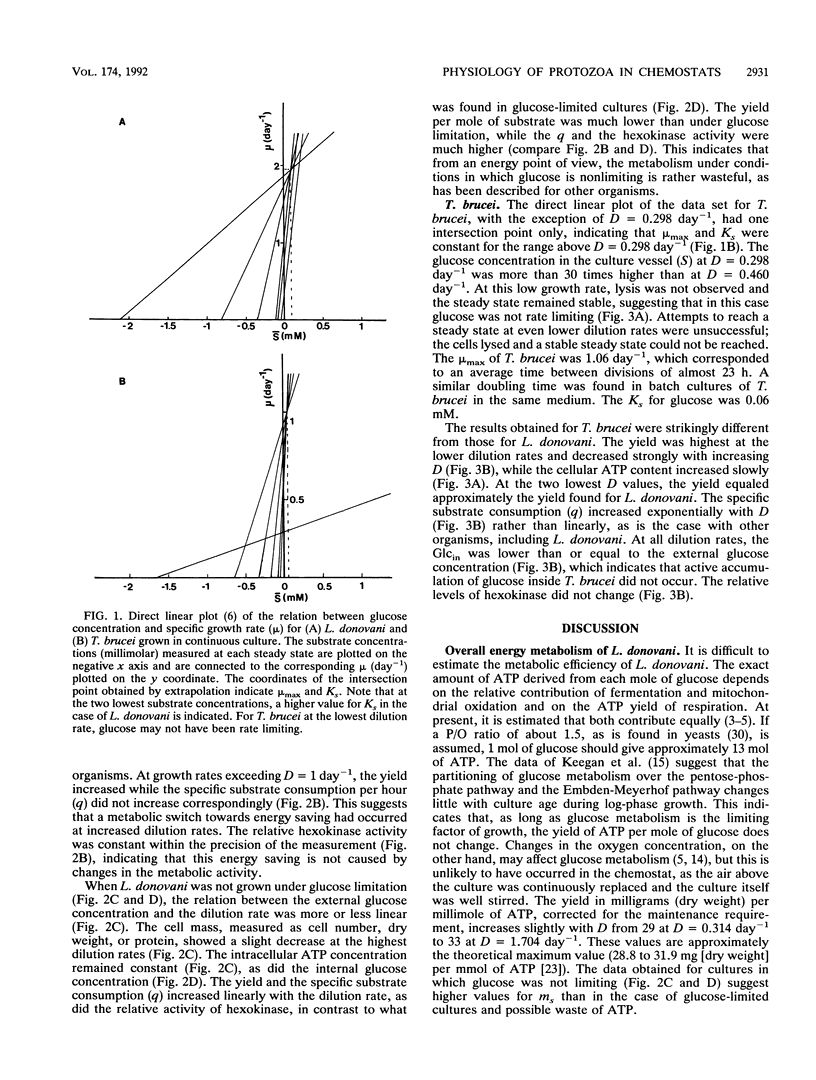
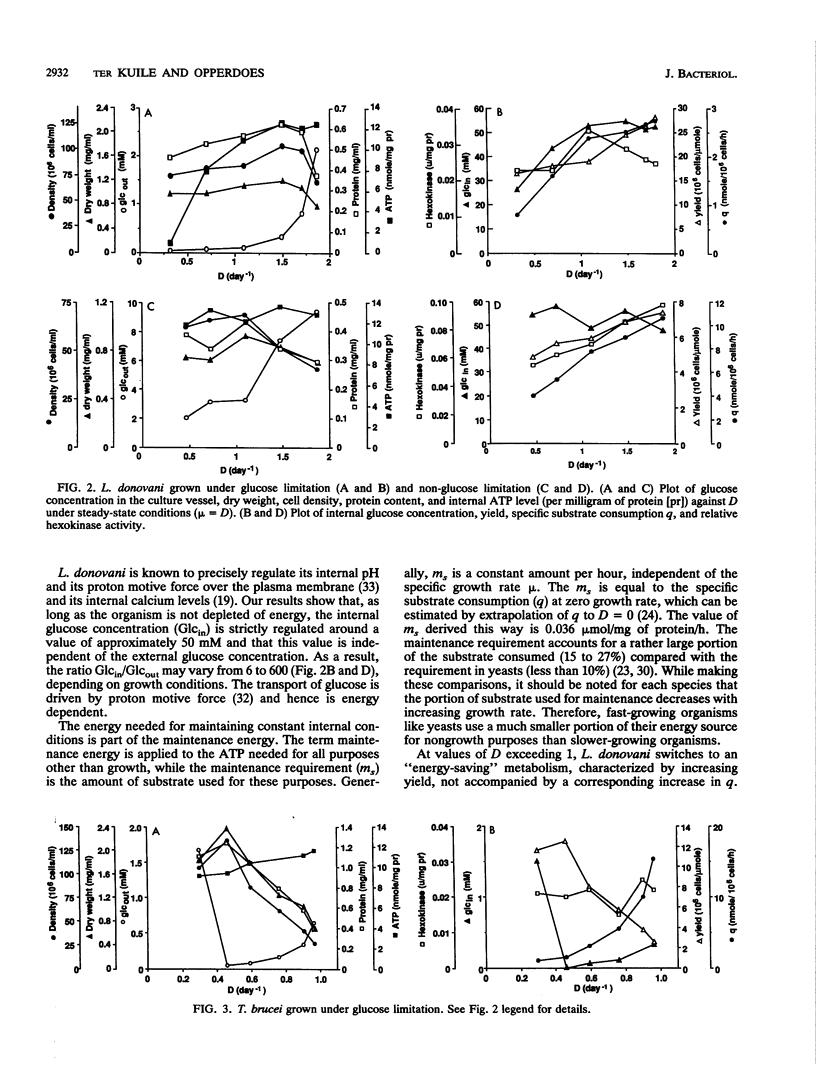
Cultures of the insect stage of the protozoan parasites Leishmania donovani and Trypanosoma brucei were grown in chemostats with glucose as the growth rate-limiting substrate. L. donovani has a maximum specific growth rate (mu max) of 1.96 day-1 and a Ks for glucose of 0.1 mM; the mu max of T. brucei is 1.06 day-1 and the Ks is 0.06 mM. At each steady state (specific growth rate, mu, equals D, the dilution rate), the following parameters were measured: external glucose concentration (Glcout), cell density, dry weight, protein, internal glucose concentration (Glcin), cellular ATP level, and hexokinase activity. L. donovani shows a relationship between mu and yield that allows an estimation of the maintenance requirement (ms) and the yield per mole of ATP (YATP). Both the ms and the YATP are on the higher margin of the range found for prokaryotes grown on glucose in a complex medium. L. donovani maintains the Glcin at a constant level of about 50 mM as long as it is not energy depleted. T. brucei has a decreasing yield with increasing mu, suggesting that it oxidizes its substrate to a lesser extent at higher growth rates. Glucose is not concentrated internally but is taken up by facilitated diffusion, while phosphorylation by hexokinase is probably the rate-limiting step for glucose metabolism. The Ks is constant as long as glucose is the rate-limiting substrate. The results of this study demonstrate that L. donovani and T. brucei have widely different metabolic strategies for dealing with varying external conditions, which reflect the conditions they are likely to encounter in their respective insect hosts.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brun R., Schönenberger Cultivation and in vitro cloning or procyclic culture forms of Trypanosoma brucei in a semi-defined medium. Short communication. Acta Trop. 1979 Sep;36(3):289–292. [PubMed] [Google Scholar]
- Cazzulo J. J., Franke de Cazzulo B. M., Engel J. C., Cannata J. J. End products and enzyme levels of aerobic glucose fermentation in trypanosomatids. Mol Biochem Parasitol. 1985 Sep;16(3):329–343. doi: 10.1016/0166-6851(85)90074-x. [DOI] [PubMed] [Google Scholar]
- Darling T. N., Davis D. G., London R. E., Blum J. J. Carbon dioxide abolishes the reverse Pasteur effect in Leishmania major promastigotes. Mol Biochem Parasitol. 1989 Mar 1;33(2):191–202. doi: 10.1016/0166-6851(89)90033-9. [DOI] [PubMed] [Google Scholar]
- Darling T. N., Davis D. G., London R. E., Blum J. J. Products of Leishmania braziliensis glucose catabolism: release of D-lactate and, under anaerobic conditions, glycerol. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7129–7133. doi: 10.1073/pnas.84.20.7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenthal R., Game S., Holman G. D. Specificity and kinetics of hexose transport in Trypanosoma brucei. Biochim Biophys Acta. 1989 Oct 2;985(1):81–89. doi: 10.1016/0005-2736(89)90107-7. [DOI] [PubMed] [Google Scholar]
- Ellenberger T. E., Beverley S. M. Biochemistry and regulation of folate and methotrexate transport in Leishmania major. J Biol Chem. 1987 Jul 25;262(21):10053–10058. [PubMed] [Google Scholar]
- Game S., Holman G., Eisenthal R. Sugar transport in Trypanosoma brucei: a suitable kinetic probe. FEBS Lett. 1986 Jan 1;194(1):126–130. doi: 10.1016/0014-5793(86)80063-1. [DOI] [PubMed] [Google Scholar]
- Gruenberg J., Sharma P. R., Deshusses J. D-Glucose transport in Trypanosoma brucei. D-Glucose transport is the rate-limiting step of its metabolism. Eur J Biochem. 1978 Sep 1;89(2):461–469. doi: 10.1111/j.1432-1033.1978.tb12549.x. [DOI] [PubMed] [Google Scholar]
- Keegan F. P., Sansone L., Blum J. J. Oxidation of glucose, ribose, alanine, and glutamate by Leishmania braziliensis panamensis. J Protozool. 1987 May;34(2):174–179. doi: 10.1111/j.1550-7408.1987.tb03156.x. [DOI] [PubMed] [Google Scholar]
- Keegan F., Blum J. J. Effects of oxygen concentration on the intermediary metabolism of Leishmania major promastigotes. Mol Biochem Parasitol. 1990 Mar;39(2):235–245. doi: 10.1016/0166-6851(90)90062-q. [DOI] [PubMed] [Google Scholar]
- Njogu R. M., Whittaker C. J., Hill G. C. Evidence for a branched electron transport chain in Trypanosoma brucei. Mol Biochem Parasitol. 1980 Mar;1(1):13–29. doi: 10.1016/0166-6851(80)90038-9. [DOI] [PubMed] [Google Scholar]
- Opperdoes F. R., Markoŝ A., Steiger R. F. Localization of malate dehydrogenase, adenylate kinase and glycolytic enzymes in glycosomes and the threonine pathway in the mitochondrion of cultured procyclic trypomastigotes of Trypanosoma brucei. Mol Biochem Parasitol. 1981 Dec 31;4(5-6):291–309. doi: 10.1016/0166-6851(81)90062-1. [DOI] [PubMed] [Google Scholar]
- Philosoph H., Zilberstein D. Regulation of intracellular calcium in promastigotes of the human protozoan parasite Leishmania donovani. J Biol Chem. 1989 Jun 25;264(18):10420–10424. [PubMed] [Google Scholar]
- Pirt S. J. The maintenance energy of bacteria in growing cultures. Proc R Soc Lond B Biol Sci. 1965 Oct 12;163(991):224–231. doi: 10.1098/rspb.1965.0069. [DOI] [PubMed] [Google Scholar]
- RYLEY J. F. Studies on the metabolism of the protozoa. 9. Comparative metabolism of blood-stream and culture forms of Trypanosoma rhodesiense. Biochem J. 1962 Oct;85:211–223. doi: 10.1042/bj0850211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein S., Böhlen P., Stone J., Dairman W., Udenfriend S. Amino acid analysis with fluorescamine at the picomole level. Arch Biochem Biophys. 1973 Mar;155(1):202–212. doi: 10.1016/s0003-9861(73)80022-0. [DOI] [PubMed] [Google Scholar]
- Ter Kuile B. H., Opperdoes F. R. Chemostat cultures of Leishmania donovani promastigotes and Trypanosoma brucei procyclic trypomastigotes. Mol Biochem Parasitol. 1991 Mar;45(1):171–173. doi: 10.1016/0166-6851(91)90039-9. [DOI] [PubMed] [Google Scholar]
- Ter Kuile B. H., Opperdoes F. R. Glucose uptake by Trypanosoma brucei. Rate-limiting steps in glycolysis and regulation of the glycolytic flux. J Biol Chem. 1991 Jan 15;266(2):857–862. [PubMed] [Google Scholar]
- Torri A. F., Hajduk S. L. Posttranscriptional regulation of cytochrome c expression during the developmental cycle of Trypanosoma brucei. Mol Cell Biol. 1988 Nov;8(11):4625–4633. doi: 10.1128/mcb.8.11.4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verduyn C., Stouthamer A. H., Scheffers W. A., van Dijken J. P. A theoretical evaluation of growth yields of yeasts. Antonie Van Leeuwenhoek. 1991 Jan;59(1):49–63. doi: 10.1007/BF00582119. [DOI] [PubMed] [Google Scholar]
- Zilberstein D., Dwyer D. M. Glucose transport in Leishmania donovani promastigotes. Mol Biochem Parasitol. 1984 Jul;12(3):327–336. doi: 10.1016/0166-6851(84)90089-6. [DOI] [PubMed] [Google Scholar]
- Zilberstein D., Dwyer D. M. Protonmotive force-driven active transport of D-glucose and L-proline in the protozoan parasite Leishmania donovani. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1716–1720. doi: 10.1073/pnas.82.6.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein D., Philosoph H., Gepstein A. Maintenance of cytoplasmic pH and proton motive force in promastigotes of Leishmania donovani. Mol Biochem Parasitol. 1989 Sep;36(2):109–117. doi: 10.1016/0166-6851(89)90183-7. [DOI] [PubMed] [Google Scholar]
- ter Kuile B. H., Opperdoes F. R. Mutual adjustment of glucose uptake and metabolism in Trypanosoma brucei grown in a chemostat. J Bacteriol. 1992 Feb;174(4):1273–1279. doi: 10.1128/jb.174.4.1273-1279.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


