Abstract
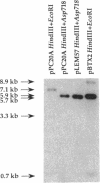
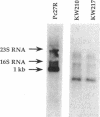
Pseudomonas syringae pv. coronafaciens, a pathogen of oats, was mutagenized with Tn5 to generate mutants defective in tabtoxin production. From a screen of 3,400 kanamycin-resistant transconjugants, seven independent mutants that do not produce tabtoxin (Tox-) were isolated. Although the Tn5 insertions within these seven mutants were linked, they were not located in the previously described tabtoxin biosynthetic region of P. syringae. Instead, all of the insertions were within the P. syringae pv. coronafaciens lemA gene. The lemA gene is required by strains of P. syringae pv. syringae for pathogenicity on bean plants (Phaseolus vulgaris). In contrast to the phenotype of a P. syringae pv. syringae lemA mutant, the Tox- mutants of P. syringae pv. coronafaciens were still able to produce necrotic lesions on oat plants (Avena sativa), although without the chlorosis associated with tabtoxin production. Northern (RNA) hybridization experiments indicated that a functional lemA gene was required for the detection of a transcript produced from the tblA locus located in the tabtoxin biosynthetic region. Marker exchange mutagenesis of the tblA locus resulted in loss of tabtoxin production. Therefore, both the tblA and lemA genes are required for tabtoxin biosynthesis, and the regulation of tabtoxin production by lemA probably occurs at the transcriptional level.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Cobianchi F., Wilson S. H. Enzymes for modifying and labeling DNA and RNA. Methods Enzymol. 1987;152:94–110. doi: 10.1016/0076-6879(87)52013-4. [DOI] [PubMed] [Google Scholar]
- Ditta G., Schmidhauser T., Yakobson E., Lu P., Liang X. W., Finlay D. R., Guiney D., Helinski D. R. Plasmids related to the broad host range vector, pRK290, useful for gene cloning and for monitoring gene expression. Plasmid. 1985 Mar;13(2):149–153. doi: 10.1016/0147-619x(85)90068-x. [DOI] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasson M. J. Indicator technique for antimetabolic toxin production by phytopathogenic species of pseudomonas. Appl Environ Microbiol. 1980 Jan;39(1):25–29. doi: 10.1128/aem.39.1.25-29.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden D. W., Kronstad J. W., Leong S. A. Mutation in a heat-regulated hsp70 gene of Ustilago maydis. EMBO J. 1989 Jul;8(7):1927–1934. doi: 10.1002/j.1460-2075.1989.tb03596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hom S. S., Uratsu S. L., Hoang F. Transposon Tn5-induced mutagenesis of Rhizobium japonicum yielding a wide variety of mutants. J Bacteriol. 1984 Jul;159(1):335–340. doi: 10.1128/jb.159.1.335-340.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabak E. M., Willis D. K. The lemA gene required for pathogenicity of Pseudomonas syringae pv. syringae on bean is a member of a family of two-component regulators. J Bacteriol. 1992 May;174(9):3011–3020. doi: 10.1128/jb.174.9.3011-3020.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. D., Gutterson N. An efficient mobilizable cosmid vector, pRK7813, and its use in a rapid method for marker exchange in Pseudomonas fluorescens strain HV37a. Gene. 1987;61(3):299–306. doi: 10.1016/0378-1119(87)90193-4. [DOI] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Keen N. T., Tamaki S., Kobayashi D., Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988 Oct 15;70(1):191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- Kinscherf T. G., Coleman R. H., Barta T. M., Willis D. K. Cloning and expression of the tabtoxin biosynthetic region from Pseudomonas syringae. J Bacteriol. 1991 Jul;173(13):4124–4132. doi: 10.1128/jb.173.13.4124-4132.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight T. J., Durbin R. D., Langston-Unkefer P. J. Role of glutamine synthetase adenylylation in the self-protection of Pseudomonas syringae subsp. "tabaci" from its toxin, tabtoxinine-beta-lactam. J Bacteriol. 1986 Apr;166(1):224–229. doi: 10.1128/jb.166.1.224-229.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight T. J., Durbin R. D., Langston-Unkefer P. J. Self-protection of Pseudomonas syringae pv. "tabaci" from its toxin, tabtoxinine-beta-lactam. J Bacteriol. 1987 May;169(5):1954–1959. doi: 10.1128/jb.169.5.1954-1959.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston-Unkefer P. L., Macy P. A., Durbin R. D. Inactivation of Glutamine Synthetase by Tabtoxinine-beta-lactam : Effects of Substrates and pH. Plant Physiol. 1984 Sep;76(1):71–74. doi: 10.1104/pp.76.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orser C., Staskawicz B. J., Panopoulos N. J., Dahlbeck D., Lindow S. E. Cloning and expression of bacterial ice nucleation genes in Escherichia coli. J Bacteriol. 1985 Oct;164(1):359–366. doi: 10.1128/jb.164.1.359-366.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Selvaraj G., Iyer V. N. Transposon Tn5 specifies streptomycin resistance in Rhizobium spp. J Bacteriol. 1984 May;158(2):580–589. doi: 10.1128/jb.158.2.580-589.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinden S. L., Durbin R. D. Glutamine synthetase inhibition: possible mode of action of wildfire toxin from Pseudomonas tabaci. Nature. 1968 Jul 27;219(5152):379–380. doi: 10.1038/219379a0. [DOI] [PubMed] [Google Scholar]
- Staskawicz B., Dahlbeck D., Keen N., Napoli C. Molecular characterization of cloned avirulence genes from race 0 and race 1 of Pseudomonas syringae pv. glycinea. J Bacteriol. 1987 Dec;169(12):5789–5794. doi: 10.1128/jb.169.12.5789-5794.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W. W. Isolation and proof of structure of wildfire toxin. Nature. 1971 Jan 15;229(5281):174–178. doi: 10.1038/229174a0. [DOI] [PubMed] [Google Scholar]
- Sykes R. B., Cimarusti C. M., Bonner D. P., Bush K., Floyd D. M., Georgopapadakou N. H., Koster W. M., Liu W. C., Parker W. L., Principe P. A. Monocyclic beta-lactam antibiotics produced by bacteria. Nature. 1981 Jun 11;291(5815):489–491. doi: 10.1038/291489a0. [DOI] [PubMed] [Google Scholar]
- Sykes R. B., Koster W. H., Bonner D. P. The new monobactams: chemistry and biology. J Clin Pharmacol. 1988 Feb;28(2):113–119. doi: 10.1002/j.1552-4604.1988.tb05734.x. [DOI] [PubMed] [Google Scholar]
- Thomas M. D., Langston-Unkefer P. J., Uchytil T. F., Durbin R. D. Inhibition of Glutamine Synthetase from Pea by Tabtoxinine-beta-lactam. Plant Physiol. 1983 Apr;71(4):912–915. doi: 10.1104/pp.71.4.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkefer C. J., London R. E., Durbin R. D., Uchytil T. F., Langston-Unkefer P. J. The biosynthesis of tabtoxinine-beta-lactam. Use of specifically 13C-labeled glucose and 13C NMR spectroscopy to identify its biosynthetic precursors. J Biol Chem. 1987 Apr 15;262(11):4994–4999. [PubMed] [Google Scholar]
- WOOLLEY D. W., PRINGLE R. B., BRAUN A. C. Isolation of the phytopathogenic toxin of Pseudomonas tabaci, an antagonist of methionine. J Biol Chem. 1952 May;197(1):409–417. [PubMed] [Google Scholar]
- Zhu Y. S., Kaplan S. Effects of light, oxygen, and substrates on steady-state levels of mRNA coding for ribulose-1,5-bisphosphate carboxylase and light-harvesting and reaction center polypeptides in Rhodopseudomonas sphaeroides. J Bacteriol. 1985 Jun;162(3):925–932. doi: 10.1128/jb.162.3.925-932.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]