Abstract
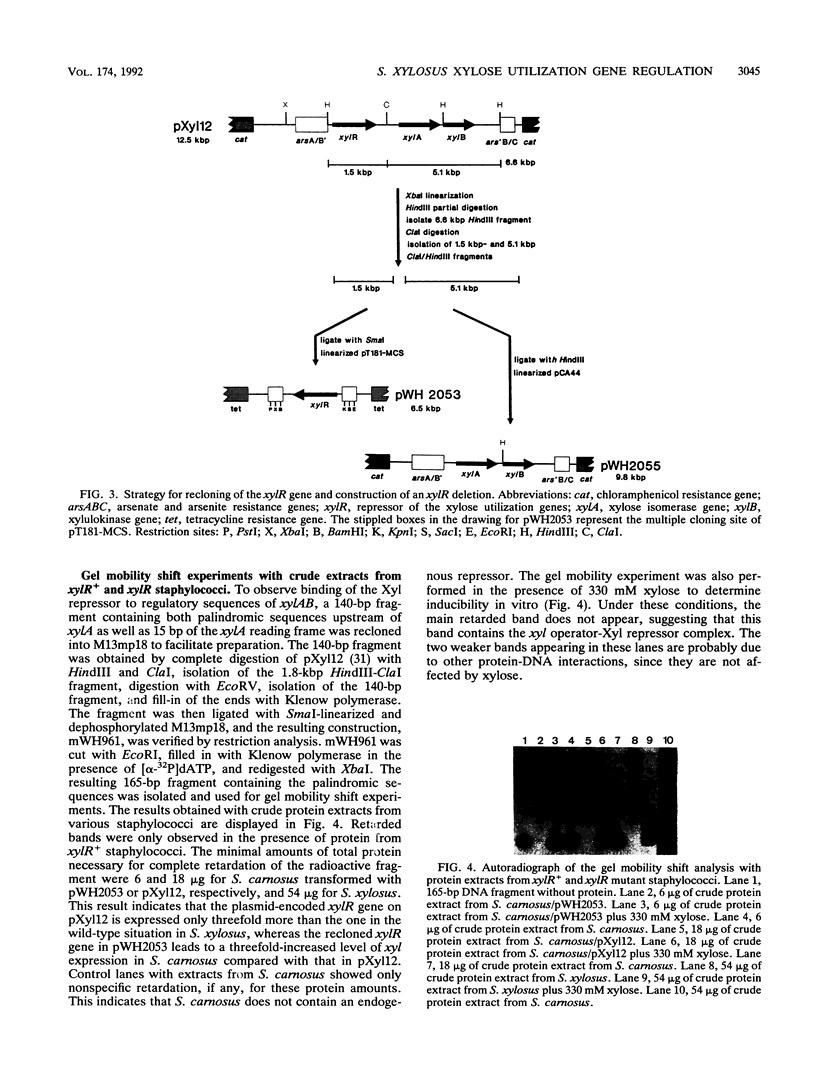
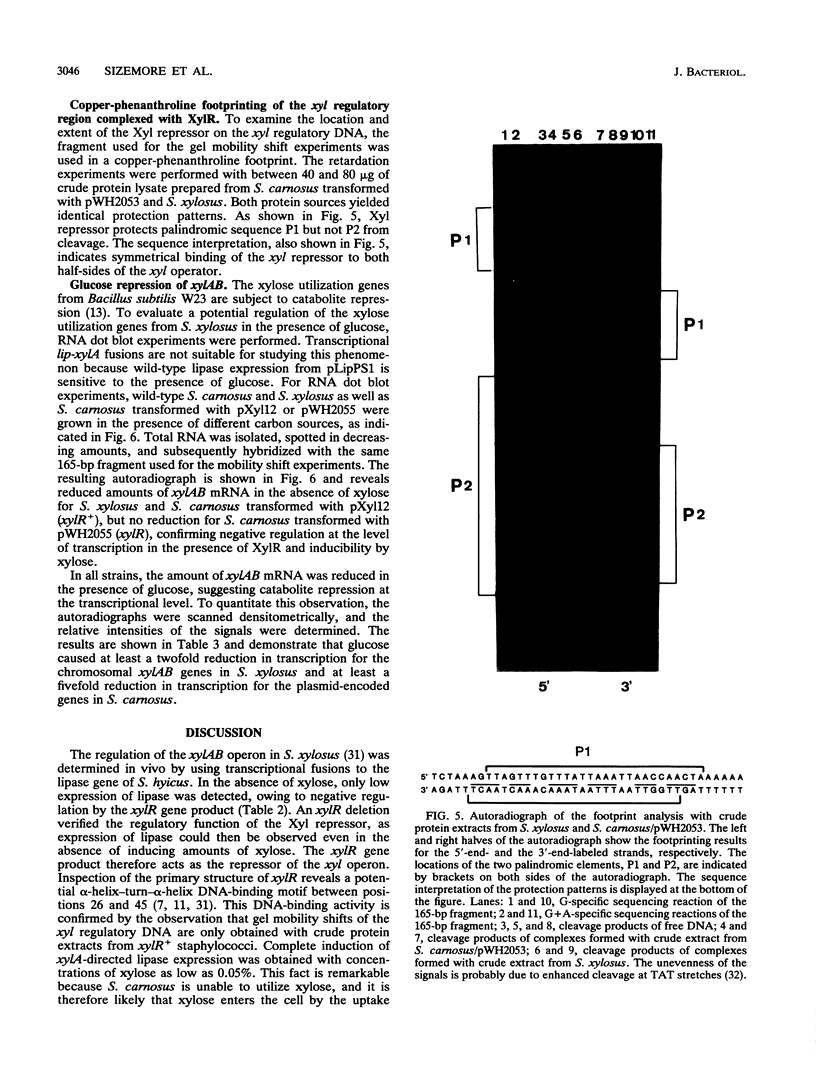
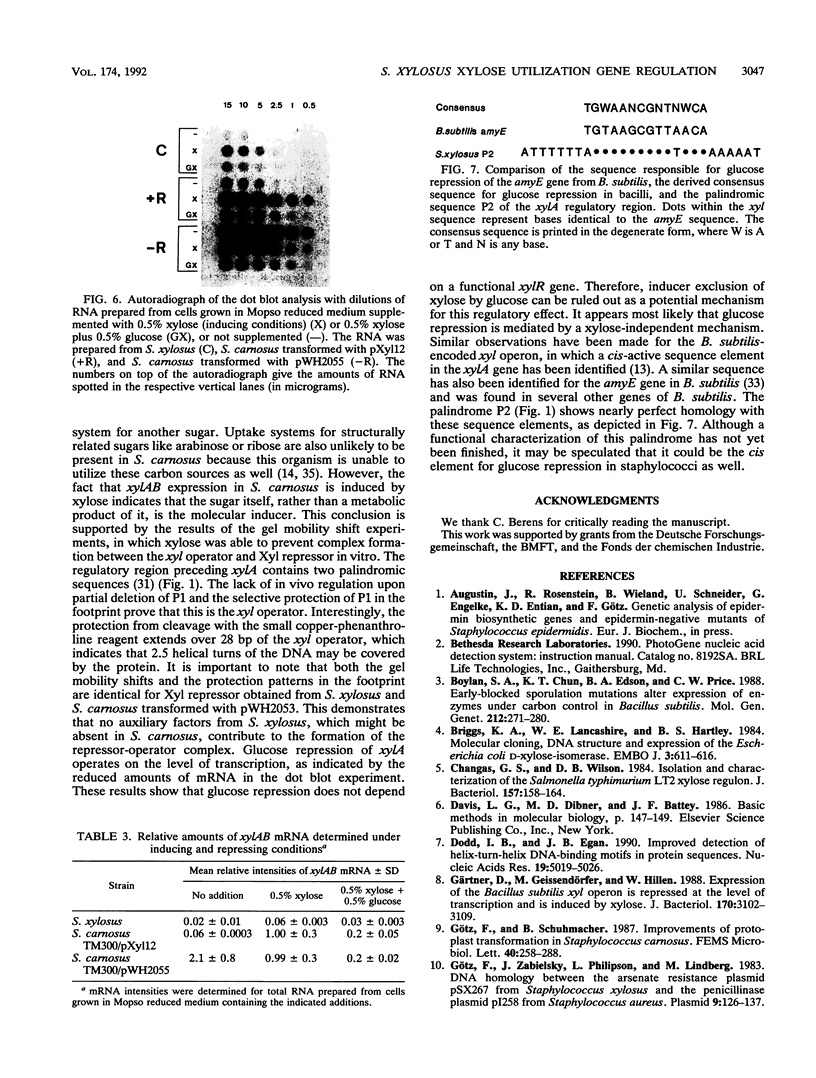
We have investigated the regulation of the operon encoding xylose utilization in Staphylococcus xylosus C2a and Staphylococcus carnosus TM300. For in vivo studies, transcriptional fusions of the xylAB regulatory region to the lipase gene from Staphylococcus hyicus were constructed. Repression of lipase activity depended on a functional xylR gene and an xyl operator palindrome downstream of the promoter, while induction was obtained in the presence of xylose. Inactivation of either xylR or the xyl operator led to constitutive expression in the absence of xylose. Crude protein extracts from xylR+ staphylococci led to gel mobility shifts of the xyl regulatory DNA in the absence but not in the presence of xylose. A copper-phenanthroline footprint of the shifted band revealed protection of 28 phosphodiesters from cleavage in each strand of the xyl operator. Thus, the Xyl repressor covers the DNA over more than 2.5 helical turns. Glucose repression of the xyl operon occurs at the level of transcription and is independent of a functional xylR gene. A potential cis-active sequence element for glucose repression is discussed on the basis of sequence similarities to respective elements from bacilli.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boylan S. A., Chun K. T., Edson B. A., Price C. W. Early-blocked sporulation mutations alter expression of enzymes under carbon control in Bacillus subtilis. Mol Gen Genet. 1988 May;212(2):271–280. doi: 10.1007/BF00334696. [DOI] [PubMed] [Google Scholar]
- Briggs K. A., Lancashire W. E., Hartley B. S. Molecular cloning, DNA structure and expression of the Escherichia coli D-xylose isomerase. EMBO J. 1984 Mar;3(3):611–616. doi: 10.1002/j.1460-2075.1984.tb01856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd I. B., Egan J. B. Improved detection of helix-turn-helix DNA-binding motifs in protein sequences. Nucleic Acids Res. 1990 Sep 11;18(17):5019–5026. doi: 10.1093/nar/18.17.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghangas G. S., Wilson D. B. Isolation and characterization of the Salmonella typhimurium LT2 xylose regulon. J Bacteriol. 1984 Jan;157(1):158–164. doi: 10.1128/jb.157.1.158-164.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gärtner D., Geissendörfer M., Hillen W. Expression of the Bacillus subtilis xyl operon is repressed at the level of transcription and is induced by xylose. J Bacteriol. 1988 Jul;170(7):3102–3109. doi: 10.1128/jb.170.7.3102-3109.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz F., Zabielski J., Philipson L., Lindberg M. DNA homology between the arsenate resistance plasmid pSX267 from Staphylococcus xylosus and the penicillinase plasmid pI258 from Staphylococcus aureus. Plasmid. 1983 Mar;9(2):126–137. doi: 10.1016/0147-619x(83)90015-x. [DOI] [PubMed] [Google Scholar]
- Harrison S. C., Aggarwal A. K. DNA recognition by proteins with the helix-turn-helix motif. Annu Rev Biochem. 1990;59:933–969. doi: 10.1146/annurev.bi.59.070190.004441. [DOI] [PubMed] [Google Scholar]
- Igarashi H., Fujikawa H., Usami H., Kawabata S., Morita T. Purification and characterization of Staphylococcus aureus FRI 1169 and 587 toxic shock syndrome exotoxins. Infect Immun. 1984 Apr;44(1):175–181. doi: 10.1128/iai.44.1.175-181.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob S., Allmansberger R., Gärtner D., Hillen W. Catabolite repression of the operon for xylose utilization from Bacillus subtilis W23 is mediated at the level of transcription and depends on a cis site in the xylA reading frame. Mol Gen Genet. 1991 Oct;229(2):189–196. doi: 10.1007/BF00272155. [DOI] [PubMed] [Google Scholar]
- Khan S. A., Novick R. P. Complete nucleotide sequence of pT181, a tetracycline-resistance plasmid from Staphylococcus aureus. Plasmid. 1983 Nov;10(3):251–259. doi: 10.1016/0147-619x(83)90039-2. [DOI] [PubMed] [Google Scholar]
- Kloos W. E. Natural populations of the genus Staphylococcus. Annu Rev Microbiol. 1980;34:559–592. doi: 10.1146/annurev.mi.34.100180.003015. [DOI] [PubMed] [Google Scholar]
- Kreutz B., Götz F. Construction of Staphylococcus plasmid vector pCA43 conferring resistance to chloramphenicol, arsenate, arsenite and antimony. Gene. 1984 Nov;31(1-3):301–304. doi: 10.1016/0378-1119(84)90226-9. [DOI] [PubMed] [Google Scholar]
- Kuwabara M. D., Sigman D. S. Footprinting DNA-protein complexes in situ following gel retardation assays using 1,10-phenanthroline-copper ion: Escherichia coli RNA polymerase-lac promoter complexes. Biochemistry. 1987 Nov 17;26(23):7234–7238. doi: 10.1021/bi00397a006. [DOI] [PubMed] [Google Scholar]
- Liebl W., Götz F. Studies on lipase directed export of Escherichia coli beta-lactamase in Staphylococcus carnosus. Mol Gen Genet. 1986 Jul;204(1):166–173. doi: 10.1007/BF00330205. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Revzin A. Gel electrophoresis assays for DNA-protein interactions. Biotechniques. 1989 Apr;7(4):346–355. [PubMed] [Google Scholar]
- Scheler A., Rygus T., Allmansberger R., Hillen W. Molecular cloning, structure, promoters and regulatory elements for transcription of the Bacillus licheniformis encoded regulon for xylose utilization. Arch Microbiol. 1991;155(6):526–534. doi: 10.1007/BF00245345. [DOI] [PubMed] [Google Scholar]
- Sizemore C., Buchner E., Rygus T., Witke C., Götz F., Hillen W. Organization, promoter analysis and transcriptional regulation of the Staphylococcus xylosus xylose utilization operon. Mol Gen Genet. 1991 Jul;227(3):377–384. doi: 10.1007/BF00273926. [DOI] [PubMed] [Google Scholar]
- Veal J. M., Merchant K., Rill R. L. The influence of reducing agent and 1,10-phenanthroline concentration on DNA cleavage by phenanthroline + copper. Nucleic Acids Res. 1991 Jun 25;19(12):3383–3388. doi: 10.1093/nar/19.12.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weickert M. J., Chambliss G. H. Site-directed mutagenesis of a catabolite repression operator sequence in Bacillus subtilis. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6238–6242. doi: 10.1073/pnas.87.16.6238. [DOI] [PMC free article] [PubMed] [Google Scholar]