Abstract
Our recent data show that in cardiac myocytes polyunsaturated fatty acids (PUFAs) are antiarrhythmic. They reduce INa, shorten the action potential, shift the threshold for excitation to more positive potentials, and prolong the relative refractory period. In this study we use patch-clamp techniques in whole-cell mode and confocal Ca2+ imaging to examine the effects of PUFAs on the voltage-gated L-type Ca2+ current (ICa,L), elementary sarcoplasmic reticulum Ca2+-release events (Ca2+-sparks), and [Ca2+]i transients in isolated rat ventricular myocytes. Extracellular application of eicosapentaenoic acid (EPA; C20:5 n − 3) produced a prompt and reversible concentration-dependent suppression of ICa,L. The concentration of EPA to produce 50% inhibition of ICa was 0.8 μM in neonatal rat heart cells and 2.1 μM in adult ventricular myocytes. While the EPA induced suppression of ICa,L, it did not significantly alter the shape of the current–voltage relation but did produce a small, but significant, negative shift of the steady-state inactivation curve. The inhibition of ICa,L was voltage- and time-dependent, but not use- or frequency-dependent. Other PUFAs, such as docosahexaenoic acid, arachidonic acid, linolenic acid, linoleic acid, conjugated linoleic acid, and eicosatetraynoic acid had similar effects on ICa,L as EPA. All-trans-retinoic acid, which had been shown to suppress induced arrhythmogenic activity in rat heart cells, also produced a significant inhibition of ICa,L. The saturated stearic acid and the monounsaturated oleic acid had no effect on ICa,L. Because both ICa,L and sarcoplasmic reticulum Ca2+-release underlie many cardiac arrhythmias, we examined the effects of EPA on ICa,L and Ca2+-sparks. While EPA suppressed both, it did not change the temporal or spatial character of the Ca2+-sparks, nor did it alter the ability of ICa,L to trigger Ca2+-sparks. We conclude that PUFAs may act as antiarrhythmic agents in vivo in normal and Ca2+-overloaded cells principally because they reduce Ca2+ entry by blocking ICa,L. Furthermore, PUFAs act directly to decrease INa and ICa,L, but indirectly to reduce the [Ca2+]i transients and [Ca2+]i-activated membrane current. Although a negative inotropic action is associated with application of PUFAs, it is clear that by reducing ICa,L, INa and Ca2+-sparks, PUFAs can reduce spontaneous extrasystoles in the heart. The mechanisms by which PUFAs act are discussed.
Keywords: eicosapentaenoic acid, docosahexaenoic acid, arachidonic acid
Several studies now have reported that the n-3 polyunsaturated marine oils prevent ischemia-induced malignant cardiac arrhythmias in animals (1–4) and probably in humans (5–7). This protective antiarrhythmic effect of ingestion of fish or fish oil has been shown to be attributable to their eicosapentaenoic acid (EPA; C20:5 n − 3) and docosahexaenoic acid (C22:6 n − 3) content as demonstrated by administering a highly concentrated fish oil preparation of free n − 3 PUFAs intravenously just before the ischemic challenge and preventing the fatal arrhythmias (4). But studies with isolated cultured neonatal rat cardiac myocytes showed that polyunsaturated fatty acids, both n − 6 and n − 3, are antiarrhythmic during Ca2+ overload (8). Arachidonic acid (AA; C20:4 n − 6), however, is anomalous in its effects. Although, as a free fatty acid, AA is also antiarrhythmic, its cyclooxygenase eicosanoids cause arrhythmias (8). The monounsaturated fatty acid, oleic acid (C18:1 n − 9), and saturated fatty acids have no antiarrhythmic effects.
Further the antiarrhythmic effect occurs quickly but only with the free fatty acid form of the PUFAs; the ethyl ester or triglyceride are not promptly antiarrhythmic (8). Presumably, the free PUFAs exert their effect by partitioning into the hospitable, hydrophobic environment of the cell membranes among the acyl chains of the phospholipids of cardiomyocytes, but a receptor-mediated signaling pathway cannot be ruled out. In any case, the effect is quickly reversed when the free PUFAs are extracted from the cells by adding delipidated BSA to the bathing medium. This observation suggests that the PUFAs are neither fully incorporated into membrane phospholipids nor covalent bound to any constituents of the myocyte to produce the antiarrhythmic effect (8).
The presence of the free PUFAs in the extracellular medium alters the electrophysiology of the cardiac myocytes and can restore electrical stability to Ca2+-overloaded cells. This apparently beneficial action of the PUFAs is associated with a reduction in cellular excitability due to a more positive threshold for the gating of INa, hyperpolarization of the resting potential, and prolongation of the relative refractory period despite shortening of the action potential (9) and a reduced INa (10). In this study we examine the role of ICa,L and sarcoplasmic reticulum (SR) Ca2+ release in the action of PUFAs on cardiac myocytes. We observe clear reductions in ICa,L and SR Ca2+ release, both important in Ca2+ overload arrhythmias (11–15). We find that the direct action of PUFAs responsible for the reduced SR Ca2+ release is on ICa,L. We discuss the possible molecular actions of PUFAs that can account for their reductions of INa, potassium currents, and ICa,L.
MATERIALS AND METHODS
Materials.
Fatty acids (obtained from Sigma) were dissolved in 100% ethanol at a concentration of 10 mM and stored under a nitrogen atmosphere at −20°C before use. The experimental concentration of fatty acids was obtained by dilution of the stocks and contained negligible ethanol, which alone had no effect on ICa. The pipette solution for recording ICa,L contained (in mM): CsCl 100, CsOH 40, MgCl2 1, CaCl2 1, EGTA 11, Hepes 10, MgATP 5 (pH, 7.3) or contained (whenever [Ca2+]i was measured) CsCl 130, fluo-3 0.1, Hepes 10, MgCl2 0.33, tetraethylammonium chloride 20, MgATP 4 (pH, 7.2). The bathing solution contained (in mM): NaCl 137, CsCl 5, MgCl2 1, CaCl2 2, Hepes 10, glucose 10 (pH, 7.4) for the measurements in neonatal heart cells. The experiments in adult myocytes used external solutions containing (in mM) NaCl 135, MgCl2 1, CsCl20, glucose 10, Hepes 10, 4-aminopyridine 3, CaCl2 1 (pH, 7.4). In some cases, N-methyl d-glucamine (120 mM) replaced the NaCl (noted in text).
Culture of Neonatal Rat Ventricular Myocytes.
Primary cultures of ventricular myocytes were prepared from 1-day-old neonatal rats with a commercial isolation kit (Worthington). This kit uses purified enzyme preparations to produce separated cells, which were seeded onto glass coverslips (3 mm diameter) and beat spontaneously. Cells were incubated at 37°C in air with 5% CO2 added and 98% relative humidity (model 3123, Forma Scientific, Marietta, OH). The culture medium was changed every other day. Ventricular myocytes used for patch-clamp experiments were 3 to 5 days in culture.
Isolation of Adult Rat Ventricular Myocytes.
The methods used to isolate single left ventricular myocytes of adult rats (males with 200 to 300 g of body weight, Sprague–Dawley; Charles River Breeding Laboratories) was similar to those previously described (13, 16). Briefly, the heart was rapidly excised from an anesthetized rat (using inhaled ethyl ether or i.p. pentobarbital), and the aorta was quickly connected to a modified Langendorf system and perfused through the coronary arteries with modified Tyrode’s solutions containing very low levels of [Ca2+] to remove blood, and then enzymes (including collagenase (CLS 2, Worthington) and protease (Sigma) were added to the perfusion solution. After enzymatic treatment (10–25 min), the hearts were washed in an enzyme-free solution, the heart was cut into small pieces, and cells were shaken loose. Extracellular Ca2+ was increased to 1 mM, and cells were kept at room temperature until use.
Recording of ICa,L, the Ca2+ Current.
Standard patch-clamp methods in whole-cell configuration were used (14, 17, 19) to record membrane current in cultured neonatal and adult rat cardiac myocytes. Electrodes had uncompensated resistance of between 0.5 and 4 MΩ. For adult cells we used only the lower resistance electrodes. Current recordings were made from the same myocyte before, during, and after exposure to fatty acids using an Axopatch 1D or 200A patch clamp amplifier (Axon Instruments, Foster City, CA). Currents were filtered at 2 kHz and analyzed using PClamp software. Various concentrations of PUFAs were applied by a fast puffing system or a rapid change bath. All experiments were performed at a room temperature of 21–23°C.
Ca2+-Sparks, Ca2+ Imaging [Ca2+]i Measurements.
To a standard pipette solution, we added 100 μM fluo-3 (see above). An MRC 600 confocal microscope coupled to a Nikon Diaphot microscope with a Zeiss 63× 1.25 NA oil immersion lens was used to image cardiac myocytes while simultaneously patch-clamping the cells in whole-cell mode. Ca2+-sparks were seen as local increases of [Ca2+]i from the resting level of about 100 nM to a peak level of about 300 nM and were imaged using line-scan image acquisition at rates between 2–6 ms per line (13, 14, 17, 18). The time-to-peak [Ca2+]i for the observed Ca2+-sparks was similar to those reported earlier (10 ms), and the half-time of decay was also about the same (20 ms) as was the size of the Ca2+-spark at the half-peak (about 2 μm).
Data Analysis. Ca2+ current.
The amplitude of ICa,L was measured as the difference between the peak inward current and the current at the end of the depolarizing pulse. Current density, pA/pF, of ICa,L of single ventricular myocytes was calculated by dividing the amplitude of the peak current by the cell membrane capacitance. The relative current of ICa,L after the treatment of PUFAs was calculated as I(PUFAs)/I(Control) from the same cell. Data were analyzed by ANOVA or by paired or unpaired Student’s t test. P < 0.05 was considered as statistically significant difference. All data are presented as mean ± SEM.
Ca2+-sparks.
Ca2+-sparks were identified by their size, elevation of [Ca2+]i above the background signal, and kinetics (see refs. 13, 15, and 17). All image processing used IDL software (Research System, Boulder, CO).
RESULTS
Voltage-Gated L-Type Ca2+ Currents, ICa,L, Are Suppressed by EPA The actions of EPA on ICa,L were examined in adult rat ventricular myocytes (see Fig.
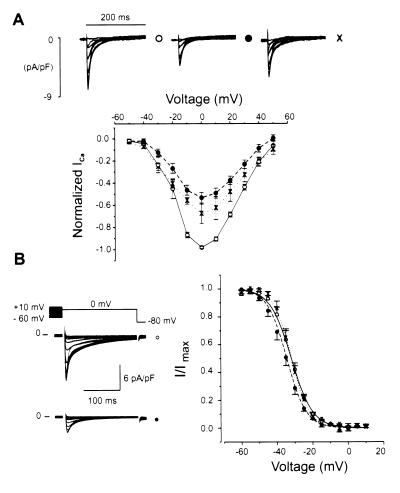
1) and in cultured neonatal rat ventricular myocytes. In adult ventricular myocytes, the following voltage protocol was applied. From a holding potential at the normal resting potential of −80 mV (to maintain normal intracellular [Ca2+]i), a slow ramp depolarization to −60 mV was applied to inactivate INa, followed by test depolarizations of 200 ms to elicit ICa,L. The application of EPA (1.5 μM) reduced ICa,L density to about half of its control level, and during the washout partial recovery was achieved. While the shape of the current–voltage relationship was not obviously changed by EPA nor was the apparent reversal potential (around +60 mV), a small (3.33 ± 0.4 mV) statistically significant negative shift of the ICa,L inactivation curve was observed. This shift was reversed by the washout of EPA.
EPA application to cultured neonatal rat ventricular myocytes at 1.0 μM concentration produced results similar to those seen in Fig. 1 despite the established differences in adult and neonatal cardiac myocyte morphology and biochemistry. Compared with the brick-like shape of the adult rat ventricular myocytes, cultured neonatal rat ventricular myocytes are thin, tentacled round and unstriated cells with reduced volume and no transverse tubules. Despite the smaller surface area, ICa,L density is greater (19). As is well-established in the literature, the resting potential in the neonatal ventricular myocyte is more positive and the cells are spontaneously active. The spontaneous activity depends largely on the interactions of inward INa and ICa,L and outward currents (dominated by several K currents) with little or no “pacemaker current,” If. The average capacitance of neonatal rat single ventricular myocytes was 51.8 ± 2.7 pF (n = 51), which is consistent with our previous report (10). The current density of peak ICa,L elicited by single-step pulse from −40 to 0 mV was 6.6 ± 0.4 pA/pF (n = 51) in the neonatal cardiomyocytes bathed in 2 mM Ca2+ Tyrode solution. The current-voltage relation and the steady-state inactivation of ICa,L in adult rat ventricular myocytes were the same as those observed in neonatal rat ventricular myocytes.
Figure 1.
Suppressant effect of EPA on the voltage-activated L-type Ca2+ current in adult rat ventricular myocytes. (A) Superimposed current density traces were elicited by 200-ms pulses with 10-mV increments from a potential of −50 mV to 50 mV. From a holding potential of −80 mV, brief depolarizations were applied to maintain a constant intracellular Ca2+ load. To elicit a test pulse, a slow ramp from −80 to −60 mV was applied, and the membrane potential was held at −60 mV for 50 ms before the test depolarization was applied. Current records under control conditions (open circles), 1.5 μM EPA (filled circles), and re-control (crosses) are shown (Upper) from a typical cell while averaged normalized current density-voltage relationships are shown (Lower) for control (n = 11), 1.5 μM EPA (n = 11) and washout (n = 5) are shown. The current density values were normalized to the maximum level observed under control conditions for each experiment. (B) Effects of EPA on the steady-state inactivation curve for ICa,L adult rat ventricular myocytes. Holding potential of −80 mV with prepulses to maintain constant SR Ca2+ load as in A. A slow depolarizing ramp was applied from −80 to −60 mV to inactivate INa. The membrane potential was immediately changed to the test potential for 1 s to produce a steady-state inactivation of ICa before stepping to 0 mV for 200 ms to assess ICa. (Left) Superimposed original Ca2+ current density records for cells exposed to no EPA (control, open circles) and for cells exposed to 10 μM EPA (closed circles). (Right) Normalized steady-state inactivation of peak ICa showing control (open circles) with fitting parameters of V0.5 = −31.97 ± 1.1 mV and K = 5.2 ± 0.26 (n = 11), 10 μM EPA (filled circles) with fitting parameters of V0.5 = −34.53 ± 1.1 mV and K = 5.11 ± 0.15 (n = 11) and washout (crosses) with fitting parameters of V0.5 = −31.86 ± 0.9 mV and K = 4.9 ± 0.13 (n = 5). Control and EPA curves are significantly different (P < 0.05).
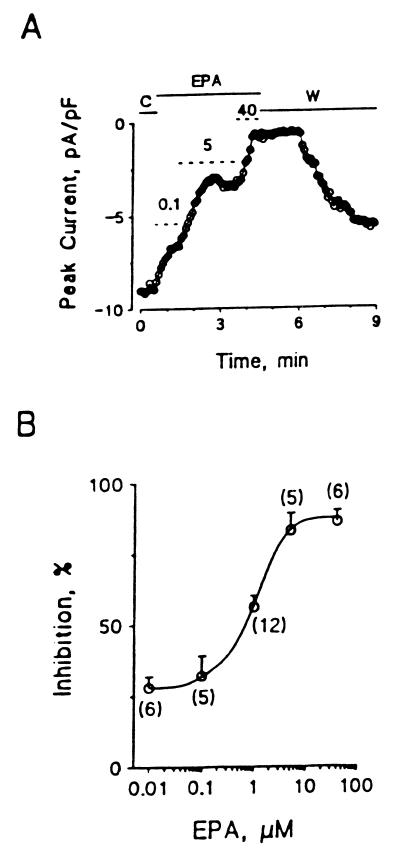
The time course of EPA-induced suppression of ICa,L in cultured neonatal rat ventricular myocytes with various EPA concentrations ranging from 0.1 μM to 40 μM is rapid, occurring in seconds. The time-course of the washout is also rapid. Fig. 2 summarizes the concentration dependence of the EPA-induced suppression of ICa,L in cultured neonatal rat ventricular myocytes. The concentration of EPA to produce 50% inhibition of ICa,L was 0.8 μM in these neonatal cells and 2.1 in adult cardiac myocytes. In neonatal heart cells ICa,L was almost completely inhibited when the concentration of EPA was above 5 μM.
Figure 2.
Inhibition of ICa,L by EPA in cultured neonatal rat ventricular myocytes. (A) Time course of the inhibitory effect of EPA on the peak ICa,L. The dotted short lines marked with 0.1, 5, and 40 represent the periods of extracellular puffing 0.1, 5, and 40 μM EPA. C, control; EPA, application of EPA; W, washout of EPA with the bath solution alone. (B) The concentration-dependent curve of EPA suppression of ICa,L. The number in parentheses represents individual ventricular myocytes treated with various concentrations of EPA. The IC50 of EPA is 0.8 μM.
Effect of Holding Potential on Inhibition of ICa,L by EPA in Na+-Free Medium.
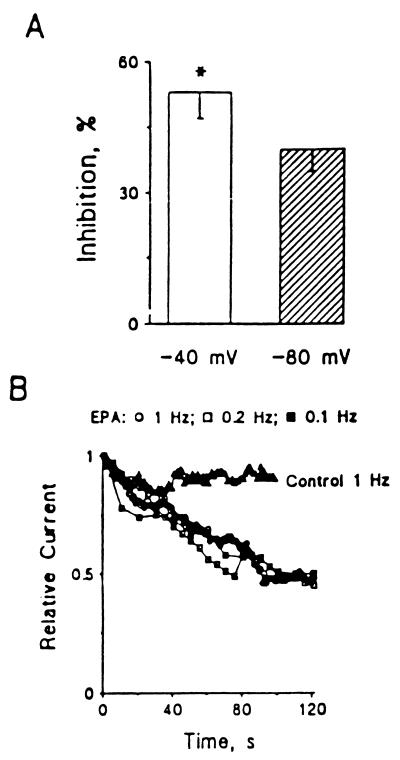
A series of experiments was done with the holding potential at either −40 mV or −80 mV in cultured neonatal rat ventricular myocytes. In these experiments 137 mM Na+ in the bath solution was replaced with 120 mM N-methyl-d-glucamine to block current through the sodium channel from contaminating ICa,L. Changing the holding potential from −40 to −80 mV did not alter the current-voltage relation ICa,L in the Na+-free bath solution. Compared with the amplitude of peak ICa,L evoked by single-step pulses from −40 to 0 mV, ICa,L elicited by pulses from −80 to 0 mV was insignificantly enhanced by 5.4 ± 3.5% (n = 6, P > 0.05). Extracellular application of 1 μM EPA produced a significantly greater suppression of ICa,L when elicited from the holding potential −40 to 0 mV than from −80 to 0 mV (53 ± 6% compared with 40 ± 5% inhibition, respectively; n = 6, P < 0.05). As changing the holding potential did affect the results, the EPA-induced suppression of ICa is voltage-dependent in cultured neonatal ventricular myocytes (Fig. 3A).
Figure 3.
Voltage- and time-dependent suppression of Ca2+ currents by EPA in cultured neonatal rat ventricular myocytes. (A) x axis represents the holding potentials. y axis represents the relative peak Ca2+ currents evoked by depolarizing pulses from −40 or −80 mV to 0 mV. (B) Time-dependent inhibition of Ca2+ currents in cultured neonatal rat ventricular myocytes. Ca2+ currents were elicited by a train of depolarizing pulses from a holding potential of −40 mV to 0 mV after a 1- to 2-min rest. Relative current was calculated by ICa,L(EPA)/ICa,L(control) recorded from the same myocyte. ▵, control, 1 Hz, n = 4; ○, 1 μM EPA, 1 Hz, n = 4; □, 1 μM EPA, 0.2 Hz, n = 3; ▪, 1 μM EPA, 0.1 Hz, n = 5.
Time-Course of the Inhibitory Actions of EPA on ICa,L.
To test whether the EPA-induced suppressant effect of ICa,L takes time to develop, as we observed previously in the study of the blocking effect of EPA on INa (10), we examined ICa,L after the addition of EPA. Additionally, we examined the effect of changing the frequency of depolarizing pulses on the action of EPA to suppress ICa,L. Ca2+ currents were elicited by a train of depolarizing pulses from a holding potential of −40 to 0 mV starting at time 0, after a 1- to 2-min rest. Fig. 3B shows that the peak ICa,L was decreased by 9 ± 10% (n = 4, P > 0.05) of the control after a 90-pulse train stimulation at 1 Hz. Application of a train of depolarizing pulses at frequencies of 0.2 or 0.1 Hz also had no significant effects on ICa,L in cultured neonatal cardiomyocytes (data not shown). Extracellular puffing 1 μM EPA produced a maximal 50 to 55% suppression of ICa,L in neonatal rat ventricular myocytes with a train stimulation at 0.1 to 1 Hz. The time required to reach the same maximal level of inhibition of ICa,L was well superimposed after application of EPA (Fig. 3B). Thus, the suppression of ICa,L by EPA developed over time but was not frequency- or use-dependent.
Effects of Other Unsaturated and Saturated Fatty Acids on ICa,L in Cultured Neonatal Rat Ventricular Myocytes.
Table 1 summarizes the effects of other fatty acids, such as docosahexaenoic acid, eicosatetraynoic acid, linolenic acid, linoleic acid, oleic acid, and stearic acid on ICa,L of cultured neonatal rat ventricular myocytes. Docosahexaenoic acid, linolenic acid, AA, and linoleic acid at 5 μM concentration produced a significantly inhibitory effect on ICa,L, which is similar to the effect of EPA. Eicosatetraynoic acid (5 μM) significantly inhibited ICa,L, but its potency on ICa,L was much less (P < 0.01) than that of EPA. All these PUFAs had similar effects on the steady-state inactivation of the calcium channel as EPA, which caused 3 to 5 mV shift to negative potentials at the V1/2 point. In contrast, extracellular application of 5 μM of oleic acid, a monounsaturated fatty acid or the saturated fatty acid, stearic acid, had neither a significant effect on ICa,L nor on the steady-state inactivation of the channel. It is interesting that a conjugated linoleic acid had a similar effect on ICa,L, but its potency was significantly less than that of EPA (Table 1).
Table 1.
Comparison of the suppressant effects of fatty acids on Ca2+ channels in cultured neonatal rat ventricular myocytes
| Fatty acid | n | μM | % inhibition | P |
|---|---|---|---|---|
| EPA | 5 | 5 | 83 ± 6 | <0.01 |
| DHA | 6 | 5 | 62 ± 6 | <0.01 |
| LNA | 5 | 5 | 77 ± 8 | <0.01 |
| AA | 6 | 5 | 76 ± 7 | <0.05 |
| LA | 6 | 5 | 76 ± 7 | <0.01 |
| CLA | 5 | 5 | 44 ± 4 | <0.01 |
| ETYA | 5 | 5 | 45 ± 6 | <0.01 |
| SA | 7 | 5 | 13 ± 6 | >0.05 |
| OA | 6 | 5 | 13 ± 10 | >0.05 |
| RA | 6 | 10 | 55 ± 7 | <0.05 |
Values represent the mean percent inhibition of ICa ± SEM. Currents were elicited by depolarizing pulses from a holding potential of −40 mV to 0 mV every 5 s. Statistical significance was tested between the peak amplitudes of ICa recorded in the absence and presence of fatty acid. n, the number of individual myocytes exposed to each fatty acid. DHA, docosahexaenoic acid; LNA, linolenic acid; LA, linoleic acid; CLA, conjugated linoleic acid; ETYA, eicosatetraynoic acid; SA, stearic acid; OA, oleic acid; RA, all-trans-retinoic acid.
It has been shown that all-trans-retinoic acid has protective effects against cardiac arrhythmias induced by isoproterenol, lysophosphatidylcholine, or ischemia and reperfusion (20). In the present study we evaluated the possibility that all-trans-retinoic acid has the same effects on ICa,L as other PUFAs. The observed inhibitory action on ICa,L may contribute importantly to its antiarrhythmic effect. Table 1 indicates that although less potent than EPA (P < 0.01), the amplitude of peak ICa,L was significantly reduced after exposure of the myocytes to all-trans-retinoic acid.
Suppression of ICa,L by PUFAs in Adult Rat Ventricular Myocytes.
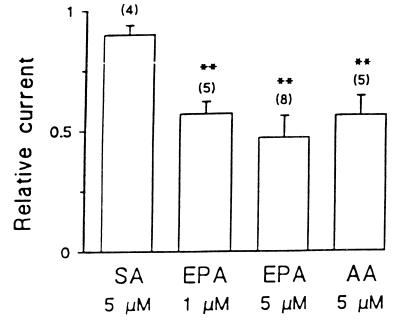
We examined the effects of EPA, AA, and stearic acid on ICa,L of left ventricular myocytes freshly isolated enzymatically from adult rat hearts. The relative potency of EPA and AA to reduce ICa,L in adult cardiac ventricular myocytes is shown in Fig. 4. Extracellular application of 1 or 5 μM EPA suppressed ICa,L to 57 ± 6% (P < 0.01, n = 5) or to 47 ± 5% (P < 0.01, n = 8) of the control level, respectively. The concentration of EPA to produce 50% inhibition of ICa,L in the adult rat ventricular myocytes was 2.1 μM, which is 2.6-fold higher than that in cultured neonatal rat cardiomyocytes (0.8 μM). Again, the saturated fatty acid, stearic acid, had no significant effects on ICa,L in adult rat ventricular myocytes.
Figure 4.
Effects of fatty acids on ICa in adult rat ventricular myocytes. ICa was elicited by 100-ms pulses of single voltage-step from a holding potential of −40 mV to 0 mV. Relative current was calculated by I Ca,L(EPA)/I Ca,L(control) recorded from the same myocyte. Each fatty acid was added to the bath solution. SA, stearic acid. ∗∗, P < 0.01; vs. the control. The number of ventricular myocytes treated with an individual fatty acid is indicated in parentheses.
Because the reported effects of AA on ICa,L of adult cardiac myocytes are conflicting (21–23), we assessed the effects of AA on ICa,L in adult rat ventricular myocytes. Extracellular application of 5 μM AA produced 44 ± 3% inhibition of peak ICa,L (from 2,142 ± 359 pA of the control to 1,206 ± 219 pA, P < 0.01) in five ventricular myocytes (Fig. 4). In the presence of AA, additional application of 1 μM isoproterenol did not antagonize the suppressant effect of AA. The averaged amplitude of peak ICa,L was 1,105 ± 218 pA (n = 5) recorded at 3 to 5 min after exposure of the myocytes to AA and isoproterenol.
Actions of EPA on Ca2+-Sparks in Adult Cardiac Myocytes.
Ca2+ signaling in adult cardiac myocytes underlies excitation-contraction coupling (19) by a mechanism known as calcium-induced Ca2+-release (CICR) (14). When a heart cell depolarizes, the brief opening of the sarcolemmal L-type Ca2+ channels permits [Ca2+]i around the channel itself to become quite high. This local [Ca2+]i increase triggers Ca2+-release from the SR through the SR Ca2+-release channels (also known as ryanodine receptors or RyRs). Thus the stochastic activation of the L-type Ca2+ channels by depolarization is responsible for triggering stochastically the RyRs to release Ca2+ by “local” CICR (14, 17, 18). The local Ca2+-release by a functional unit of excitation-contraction coupling can be observed as a Ca2+-spark in heart cells loaded with the fluorescent Ca2+-sensitive indicator, fluo-3, and viewed with a confocal microscope (13). When heart cells become “overloaded” with Ca2+, they become arrhythmogenic (see refs. 11 and 13) and produce arrhythmogenic ITI current and waves of elevated [Ca2+]i that propagate within the heart cell (15). Furthermore during Ca2+ overload the RyRs become more sensitive to the triggering process, produce an increased number of spontaneous Ca2+-sparks, and produce propagating waves of elevated Ca2+, all of which can be viewed with the confocal microscope while measuring membrane current. Thus Ca2+-sparks and their relationship to ICa,L can be used to examine the subcellular links between ICa,L, the SR, and cellular Ca2+ signaling.
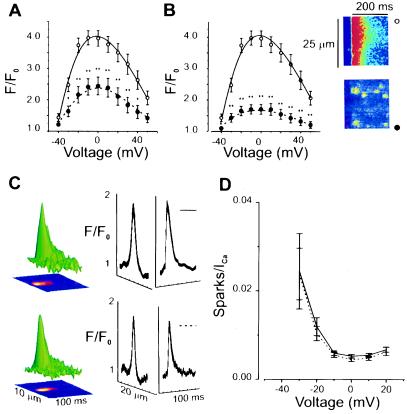
Fig. 5 A and B show that the presence of EPA (1.5 and 15 μM, respectively) markedly reduced the calcium transients induced by ICa,L with the higher concentration producing a greater reduction. To examine the subcellular nature of the actions of EPA, we signal-averaged Ca2+-sparks from control and EPA-treated single adult cardiac myocytes. The images in Fig. 5B show the line-scan images of the [Ca2+]i transient in the absence (top, ○) and presence (bottom, •) of 15 μM EPA. The reduction in SR Ca2+ release is clear from this. By contrast the examination of the individual sparks shown in Fig. 5C reveals no change, i.e., the time-constant of decay (τ) of the calcium sparks are 19.6 ± 1.4 ms (n = 46) for no EPA and 18.3 ± 1.7 ms (n = 33) after EPA application, indicating no significant change in the kinetics of Ca2+ sparks. There was also no significant difference in spatial spread of the Ca2+-sparks (see Fig. 5C). The decrease in the [Ca2+]i transient could, however, also reflect the efficacy of coupling between the L-type Ca2+ channels in the sarcolemma and the RyRs in the SR. We investigate this by examining the number of Ca2+-sparks per unit ICa,L (see e.g., ref. 17) as is shown in Fig. 5D. The solid curve (control) overlies the dashed curve (EPA) indicating that EPA reduces SR Ca2+-release only by decreasing the ICa,L that triggers SR Ca2+ release.
Figure 5.
Effects of EPA on [Ca2+]i transients and Ca2+-sparks in adult rat ventricular myocytes. Voltage protocol as in Fig. 1. (A) [Ca2+]i transient measured as peak fluorescence (F) at the test potential compared with fluorescence at −80 mV (Fo), F/Fo. Control (open circles, solid line); 1.5 μM EPA (filled circles, dashed line). (B) Same as A but 15 μM EPA. (Right) Line-scan image of a [Ca2+]i transient under control conditions at 0 mV (Upper) and after EPA (Lower). Individual Ca2+-sparks can be visualized readily after the application of EPA. (C) Signal-averaged Ca2+-spark under control conditions (Upper) τ =19.6 ± 1.4 ms (n = 46) and after the application of 15 μM EPA (Lower) τ =18.3 ± 1.7 ms (n = 33). (D) Ca2+-spark number normalized by the magnitude of ICa (EC coupling “gain function”) under control conditions, solid line (n = 9) and after 15 μM EPA, dashed line (n = 10). Significance for A and B: ∗∗, P < 0.01; ∗, P < 0.05.
DISCUSSION
The finding that only PUFAs with a free carboxyl group blocked the Na+ channels in isolated neonatal rat cardiac myocytes (10) suggested that they might be interacting with the positively charged amino acids in the S4 voltage sensor of the α-subunit of Na+ channels (24). Because this peptide segment is largely conserved in Ca2+ channels (24), it seemed likely that the PUFAs also may affect Ca2+ channels.
A reason for this study was to help explain the electrophysiologic effects of the antiarrhythmic PUFAs on cardiac myocytes. Although it had seemed possible to explain the electrophysiologic effects of the PUFAs on the basis of their blocking effects on Na+ channels (10), it was important to determine their actions on other ion channels before accepting such a simple unified action. Indeed the blocking effects PUFAs on the outward K+ currents, Ito and Ik, by the PUFAs (Y.-F.X., J.P.M., and A.L., unpublished data) are in the wrong direction to explain the observed electrophysiologic effects of the PUFAs (9). Blocking Ito and Ik would both act to prolong rather than shorten the duration of the action potentials. Also the apparent IC50 for inhibition of Ik is 20 μM compared with 4.8 μM, the IC50 for inhibition of INa (10). These findings suggest that effects of the PUFAs on K+ channels do not affect the basic electrophysiologic actions of the PUFAs on cardiomyocytes. By contrast, the sensitivity of ICa,L in ventricular myocytes to the PUFA is some 5 to 6 times greater even than on INa, suggesting a major role for inhibition of ICa,L in the electrophysiologic and antiarrhythmic actions of the PUFAs. The “vectorial” sum of the effects of PUFA on all the ion channels must account for the electrophysiologic effects observed on the myocytes.
Only the free polyunsaturated fatty acids that have been previously identified to be antiarrhythmic in the cultured spontaneously beating neonatal rat cardiomyocytes (8) affect the Ca2+ channels, as they do the Na+ channels (10). In addition, conjugated trans-linoleic acid, which has become of interest because of potential antimitotic actions (26) inhibits ICa,L. All-trans-retinoic acid, which, like the PUFAs, is specifically antiarrhythmic and shares with the PUFAs their structural requirements for an anti-arrhythmic agent (20), namely a long chain hydrocarbon, two or more C=C double bonds and a free carboxyl group at one end (8), also inhibits ICa,L, but less potently than EPA. The inhibitory effect of eicosatetraynoic acid on ICa,L, an analog of AA, but which inhibits the cyclooxygenase, lipoxygenase, and epoxygenase enzymes that oxygenated AA to form potent cell messengers (27), indicates that such AA metabolites are not playing a role in the antiarrhythmic effects of the n − 6 PUFAs.
The effects of PUFAs on the Ca2+ channels that we have observed appear to be essentially the same in the adult rat ventricular myocytes and in the neonatal rat ventricular myocytes, though the latter is more sensitive to the PUFA. The neonatal myocyte preparation has been important in our investigations because it is useful as a model for certain cardiac arrhythmias. Although this preparation consists of ventricular myocytes, which are not spontaneously active in the adult, the neonatal ventricular myocytes are intrinsically rhythmic in culture. Increased spontaneous rates are observed when ICa,L increases or when diverse potassium currents decrease. This sensitivity of the spontaneous rate is similar to the sensitivity of occurrence of “early after-depolarizations,” a kind of arrhythmia used in many model investigations (28).
We have also shown that the action of EPA to reduce ICa,L produces a reduction of the [Ca2+]i transient. While there is a reduction of the probability that Ca2+-sparks are triggered by a depolarization, the relationship between ICa,L and Ca2+-sparks is unchanged. Similarly there is no change in the kinetics of the Ca2+-spark, the elementary unit of SR Ca2+ release. From this we conclude there is no direct action of EPA on the SR Ca2+ release process or on SR Ca2+ re-uptake (29).
These effects of the free PUFAs on the ICa,L of isolated rat myocytes, as is the case with INa and IK, demonstrate that, in the absence of neuronal or hormonal stimuli, the PUFAs have a primary direct effect on membrane ion channel currents.
Kang and A.L. (30) have reported that the anti-arrhythmic PUFAs will prevent or terminate the tachyarrhythmias induced by a β-adrenergic agonist, isoproterenol, or its intracellular messenger, c-AMP. Petit-Jacques and Hartzell (31) recently have shown that the inhibitory effect of AA (C20:4 n − 6) on ICa,L was even further down stream of channel phosphorylation by protein kinase A in frog ventricular cells previously stimulated by the β-adrenergic agent isoproterenol. They concluded that the AA inhibits ICa,L by a mechanism which involves, in part, stimulation of protein phosphatase activity (29). In this study we show in isolated rat cardiomyocytes, however, that in the absence of β-adrenergic stimulation, PUFAs were still potent inhibitors of ICa,L. This is consistent with our hypothesis that the PUFAs actions result from the direct electrical consequence of their effects on ion channel proteins, not on the activity of intracellular messengers.
Pepe et al. (32) confirmed that in adult rat cardiomyocytes an earlier finding that n − 3 PUFAs inhibited the action of dihydropyridine agonists and antagonists on the L-type Ca2+ channels of neonatal rat ventricular myocytes (33). But using their whole cell patch clamp recordings, reported no inhibitory effect of the n − 3 PUFAs on the L-type Ca2+ currents per se. The finding of an inhibition of L-type Ca2+ currents in the present study is more consistent with the earlier report that n − 3 PUFAs modulate the actions of dihydropyridine agonists and antagonists in cardiomyocytes (33). The ability of the n − 3 PUFAs, shown in that study, to displace [3H]nitrendipine from its binding site at the extracellular pole of the calcium channel (34), suggests that the effects of PUFAs on ICa,L result from noncovalent binding or interaction with the protein of the calcium channel as found with the voltage-sensitive sodium channel.
Acknowledgments
This study was supported in part by the National Institute of Diabetes, Digestive and Kidney Diseases Grant DK38165 (A.L.) and National Heart, Lung, and Blood Institute Grants HL51307 (J.P.M.), HL25675 (W.J.L.), and HL36974 (W.J.L.) and by the Spanish Ministry of Education and Science (A.M.G.).
ABBREVIATIONS
- ICa,L
voltage-gated L-type Ca2+ current
- EPA
eicosapentaenoic acid
- AA
arachidonic acid
- PUFA
polyunsaturated fatty acid
- SFA
saturated fatty acid
- SR
sarcoplasmic reticulum
References
- 1.McLennan P L, Abeywardena M Y, Charnock J S. Can J Physiol Pharmacol. 1985;63:1411–1417. doi: 10.1139/y85-232. [DOI] [PubMed] [Google Scholar]
- 2.McLennan P L, Bridle T M, Abeywardena M Y, Charnock J S. Am Heart J. 1992;123:1555–1561. doi: 10.1016/0002-8703(92)90809-a. [DOI] [PubMed] [Google Scholar]
- 3.Hock C E, Beck L D, Bodine L C, Reibel D K. Am J Physiol. 1990;259:H1518–H1526. doi: 10.1152/ajpheart.1990.259.5.H1518. [DOI] [PubMed] [Google Scholar]
- 4.Billman G E, Hallaq H, Leaf A. Proc Natl Acad Sci USA. 1994;91:4427–4430. doi: 10.1073/pnas.91.10.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burr M, Gilbert J F, Holliday R M, Elwood P C, Fehily A M, Rogers S, Sweetnam P M, Deadman N M. Lancet. 1989;334:757–761. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- 6.de Logeril M, Renaud S, Mamelle N, Salen P, Martin J-L, Monjaud I, Guidollet J, Touboul P, Delaye J. Lancet. 1994;343:1454–1459. doi: 10.1016/s0140-6736(94)92580-1. [DOI] [PubMed] [Google Scholar]
- 7.Siscovick D S, Raghunathan T E, King I, Weinmann S, Wicklund K G, Albright J, Bovbjerg V, Arbogast P, Smith H, Kushi L H. J Am Med Assoc. 1995;274:1363–1367. doi: 10.1001/jama.1995.03530170043030. [DOI] [PubMed] [Google Scholar]
- 8.Kang J X, Leaf A. Proc Natl Acad Sci USA. 1994;91:9886–9890. doi: 10.1073/pnas.91.21.9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang J X, Xiao Y F, Leaf A. Proc Natl Acad Sci USA. 1995;92:3997–4001. doi: 10.1073/pnas.92.9.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao Y F, Kang J X, Morgan J P, Leaf A. Proc Natl Acad Sci USA. 1995;92:11000–11004. doi: 10.1073/pnas.92.24.11000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kass R S, Lederer W J, Tsien R W, Weingart R. J Physiol (London) 1978;281:187–208. doi: 10.1113/jphysiol.1978.sp012416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berlin J R, Cannell M B, Lederer W J. Circ Res. 1989;65:1215–1216. doi: 10.1161/01.res.65.1.115. [DOI] [PubMed] [Google Scholar]
- 13.Cheng H, Lederer W J, Cannell M B. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 14.Cannell M B, Cheng H, Lederer W J. Science. 1995;268:1045–1049. doi: 10.1126/science.7754384. [DOI] [PubMed] [Google Scholar]
- 15.Cheng H, Lederer M R, Lederer W J, Cannell M B. Am J Physiol. 1996;270:C148–C159. doi: 10.1152/ajpcell.1996.270.1.C148. [DOI] [PubMed] [Google Scholar]
- 16.Xiao Y F, MacArdle J J. J Hypertens. 1994;12:783–790. [PubMed] [Google Scholar]
- 17.Santana L F, Cheng H, Gomez A M, Cannell M B, Lederer W J. Circ Res. 1996;78:166–171. doi: 10.1161/01.res.78.1.166. [DOI] [PubMed] [Google Scholar]
- 18.Cannell M B, Cheng H, Lederer W J. Biophys J. 1994;67:1942–1956. doi: 10.1016/S0006-3495(94)80677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cannell M B, Berlin J R, Lederer W J. Science. 1987;238:1419–1423. doi: 10.1126/science.2446391. [DOI] [PubMed] [Google Scholar]
- 20.Kang J X, Leaf A. J Cardiovasc Pharmacol. 1995;26:943–948. doi: 10.1097/00005344-199512000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Kojima M, Ishima T, Taniguchi N, Kimura K, Sada H, Sperelakis N. Br J Pharmacol. 1990;99:334–339. doi: 10.1111/j.1476-5381.1990.tb14704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen C J, Bale T, Lei F, Leibowitz M D. Biophys J. 1990;57:307. (abstr.). [Google Scholar]
- 23.Huang J M, Xian H, Bacaner M. Proc Natl Acad Sci USA. 1992;89:6452–6456. doi: 10.1073/pnas.89.14.6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang J X, Leaf A. Proc Natl Acad Sci USA. 1996;93:3542–3546. doi: 10.1073/pnas.93.8.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Catterall W A. Science. 1988;242:50–61. doi: 10.1126/science.2459775. [DOI] [PubMed] [Google Scholar]
- 26.Ip C, Chin S F, Scimeca J A, Pariza M W. Cancer Res. 1991;51:6118–6124. [PubMed] [Google Scholar]
- 27.Tobias L D, Hamilton J G. Lipids. 1978;14:181–193. doi: 10.1007/BF02533870. [DOI] [PubMed] [Google Scholar]
- 28.January C T, Riddle J M. Circ Res. 1989;64:977–990. doi: 10.1161/01.res.64.5.977. [DOI] [PubMed] [Google Scholar]
- 29.Gomez A M, Cheng H, Lederer W J, Bers D M. J Physiol. 1996;496:575–581. doi: 10.1113/jphysiol.1996.sp021708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang J X, Leaf A. Biochem Biophys Res Commun. 1995;208:629–636. doi: 10.1006/bbrc.1995.1385. [DOI] [PubMed] [Google Scholar]
- 31.Petit-Jacques J, Hartzell H C. J Physiol (London) 1996;493:67–81. doi: 10.1113/jphysiol.1996.sp021365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pepe S, Bogdanov K, Hallaq H, Spurgeon H, Leaf A, Lakatta E. Proc Natl Acad Sci USA. 1994;91:8832–8836. doi: 10.1073/pnas.91.19.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hallaq H, Smith T W, Leaf A. Proc Natl Acad Sci USA. 1992;89:1760–1764. doi: 10.1073/pnas.89.5.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakayama H, Taki M, Striessnig J, Glossmann H, Catterall W A, Kanaoka Y. Proc Natl Acad Sci USA. 1991;88:9203–9207. doi: 10.1073/pnas.88.20.9203. [DOI] [PMC free article] [PubMed] [Google Scholar]