Abstract
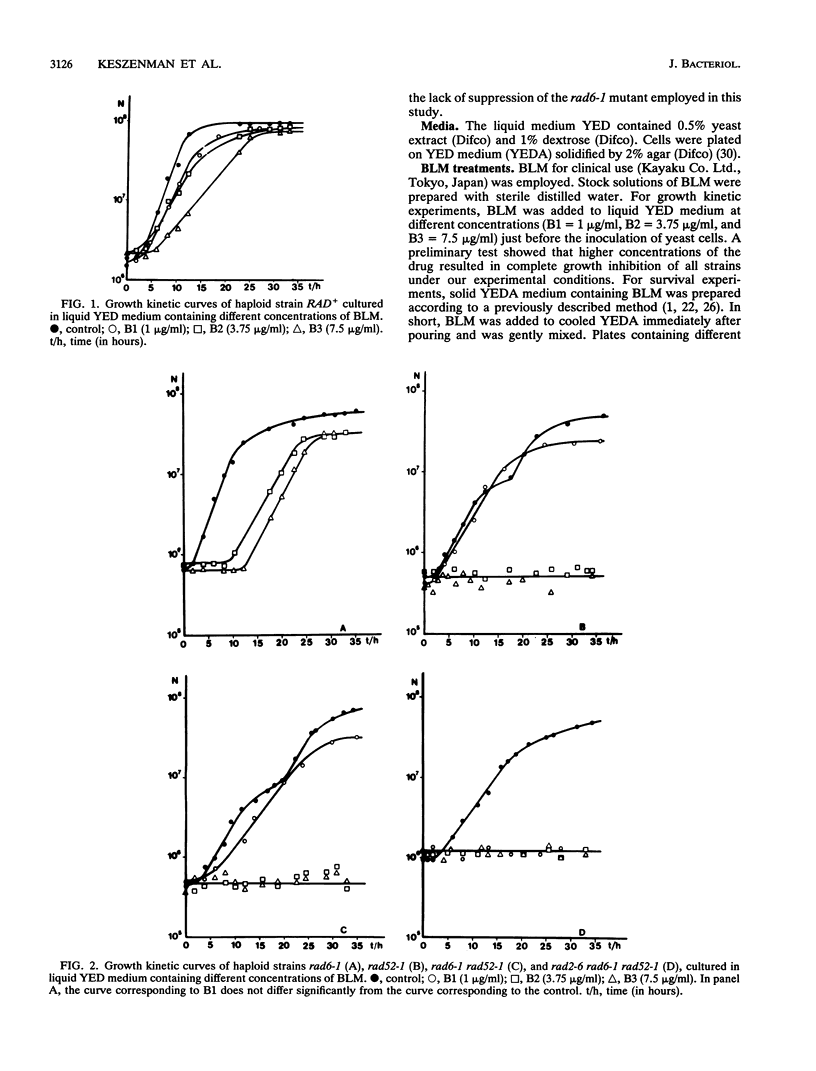
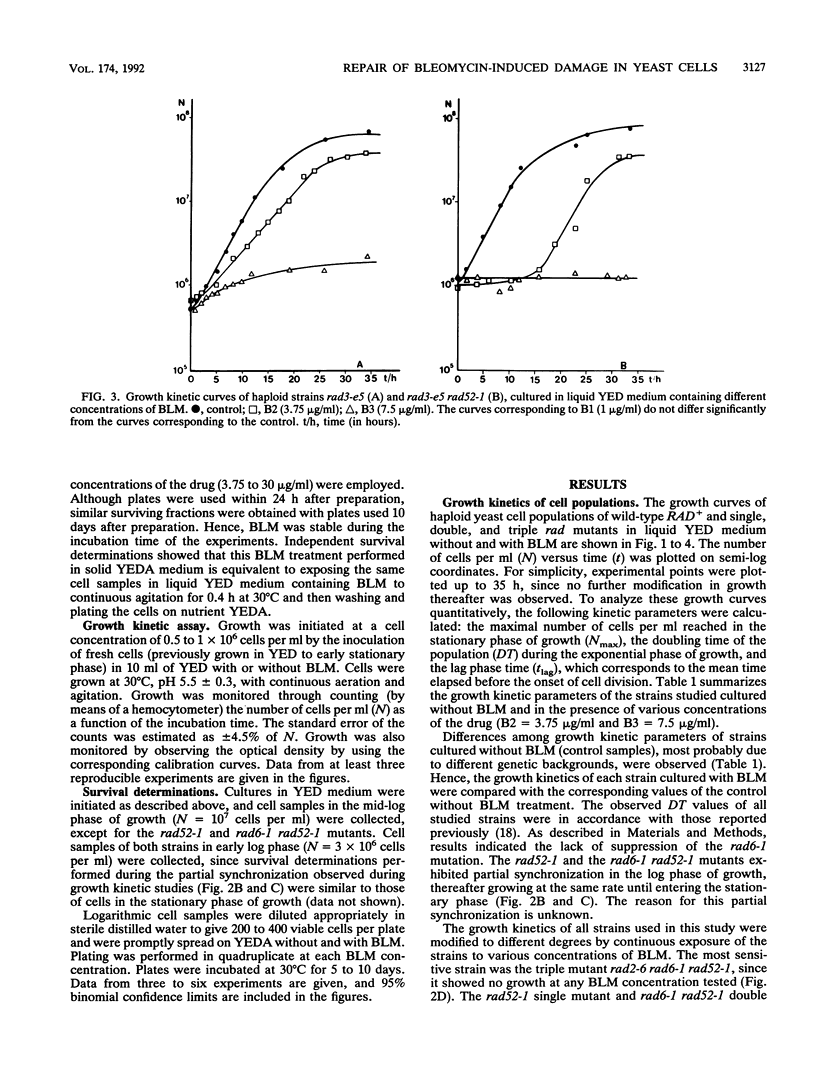
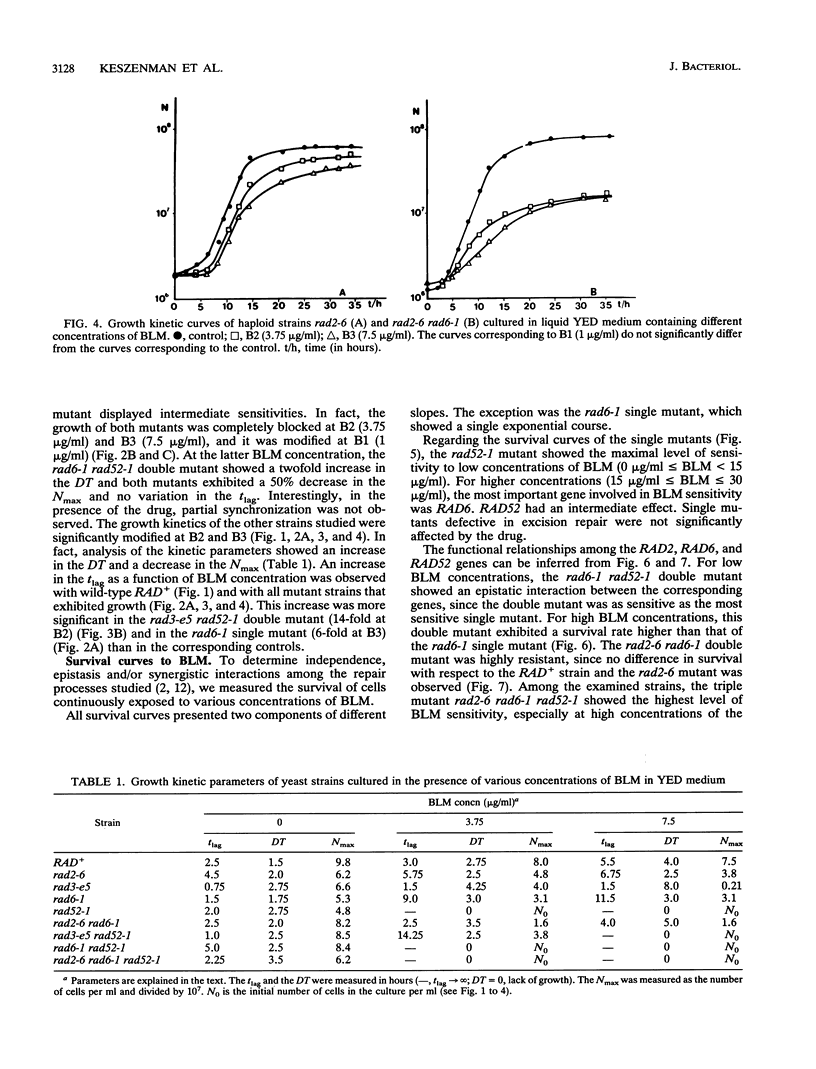
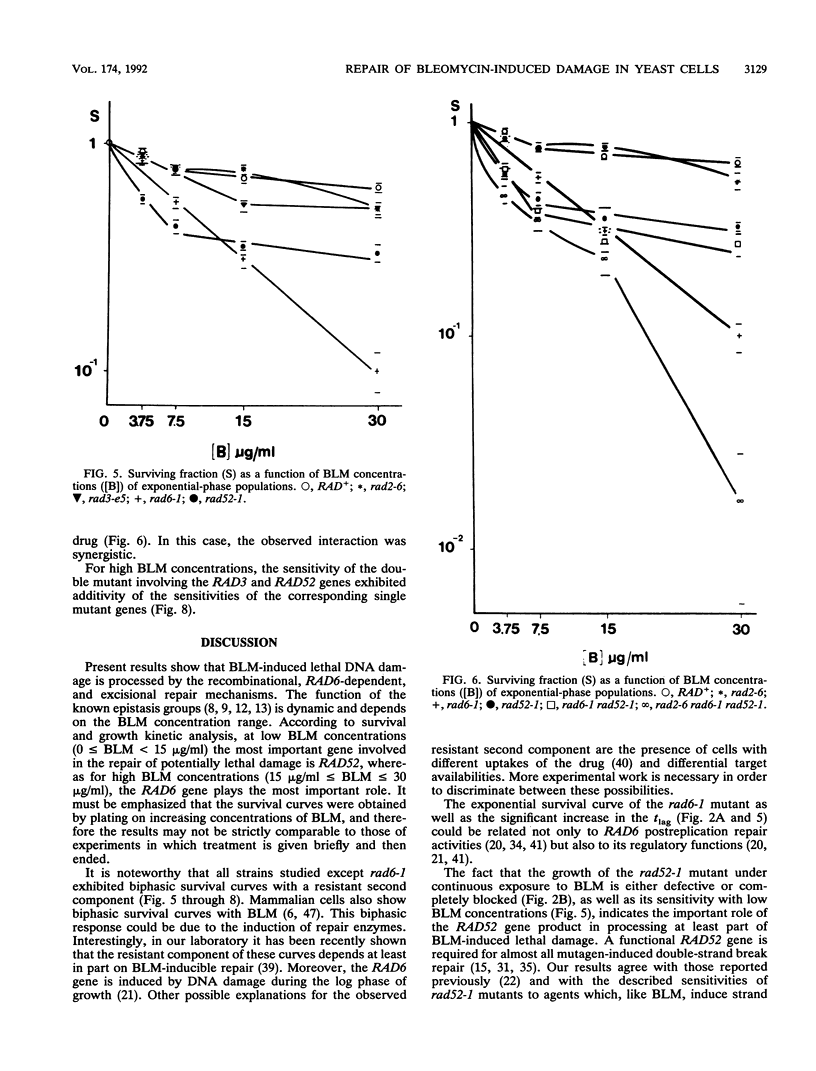
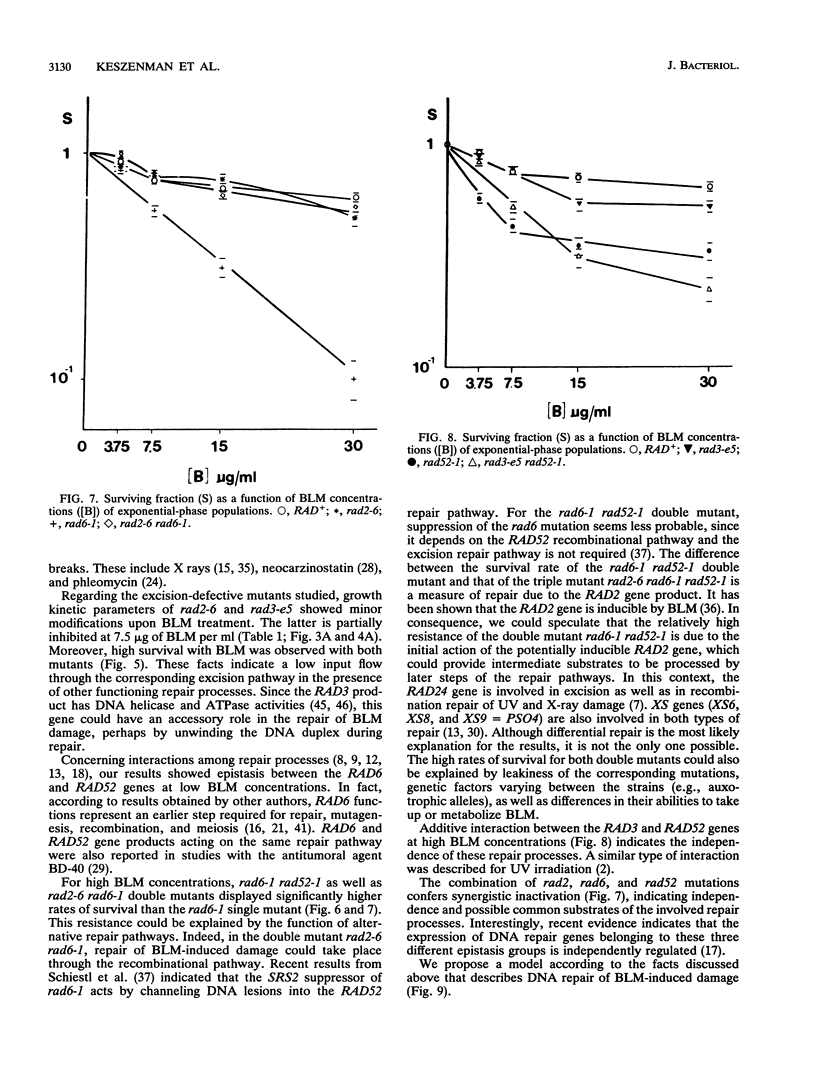
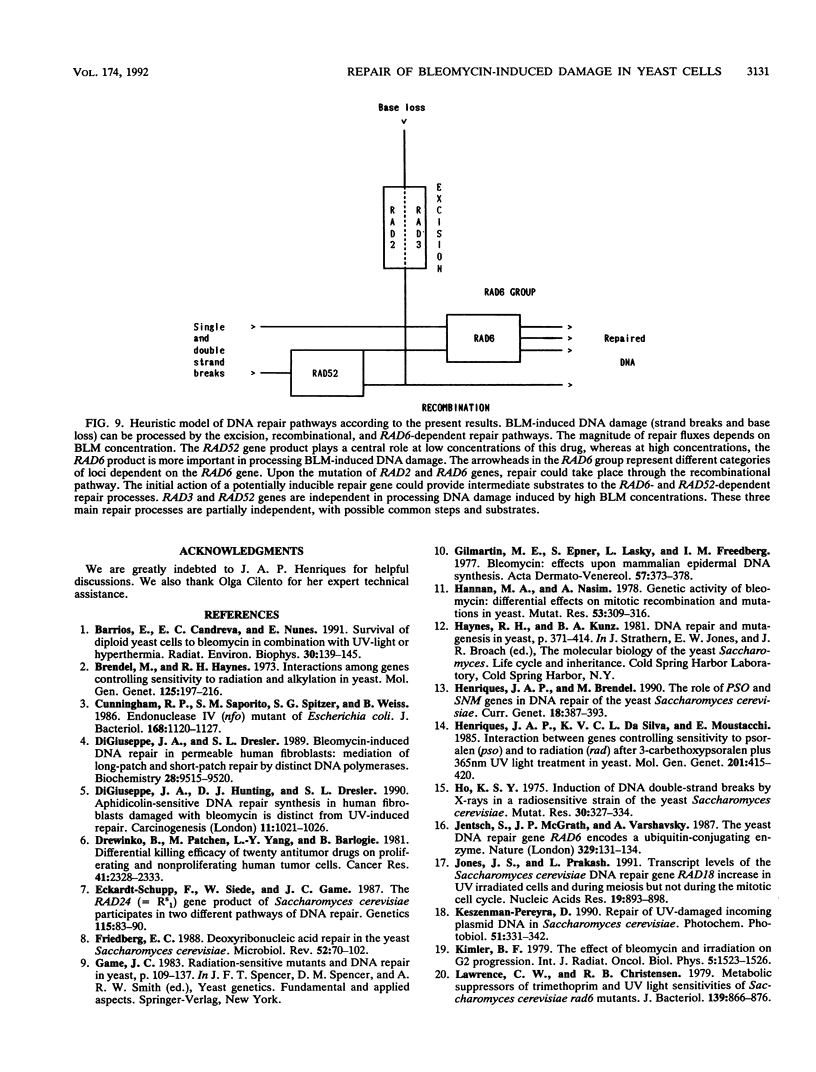
In order to analyze the roles of some repair genes in the processing of bleomycin-induced DNA damage and, especially, the interrelationships among the involved repair pathways, we investigated the potentially lethal effect of bleomycin on radiosensitive mutants of Saccharomyces cerevisiae defective in recombination, excision, and RAD6-dependent DNA repair. Using single, double, and triple rad mutants, we analyzed growth kinetics and survival curves as a function of bleomycin concentration. Our results indicate that genes belonging to the three epistasis groups interact in the repair of bleomycin-induced DNA damage to different degrees depending on the concentration of bleomycin. The most important mechanisms involved are recombination and postreplication repair. The initial action of a potentially inducible excision repair gene could provide intermediate substrates for the RAD6- and RAD52-dependent repair processes. Interaction between RAD6 and RAD52 genes was epistatic for low bleomycin concentrations. RAD3 and RAD52 genes act independently in processing DNA damage induced by high concentrations of bleomycin. The synergistic interaction observed at high concentrations in the triple mutant rad2-6 rad6-1 rad52-1 indicates partial independence of the involved repair pathways, with possible common substrates. On the basis of the present results, we propose a heuristic model of bleomycin-induced DNA damage repair.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrios E., Candreva E. C., Nunes E. Survival of diploid yeast cells to bleomycin in combination with UV-light or hyperthermia. Radiat Environ Biophys. 1991;30(2):139–145. doi: 10.1007/BF01219348. [DOI] [PubMed] [Google Scholar]
- Brendel M., Haynes R. H. Interactions among genes controlling sensitivity to radiation and alkylation in yeast. Mol Gen Genet. 1973 Sep 12;125(3):197–216. doi: 10.1007/BF00270743. [DOI] [PubMed] [Google Scholar]
- Cunningham R. P., Saporito S. M., Spitzer S. G., Weiss B. Endonuclease IV (nfo) mutant of Escherichia coli. J Bacteriol. 1986 Dec;168(3):1120–1127. doi: 10.1128/jb.168.3.1120-1127.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGiuseppe J. A., Dresler S. L. Bleomycin-induced DNA repair synthesis in permeable human fibroblasts: mediation of long-patch and short-patch repair by distinct DNA polymerases. Biochemistry. 1989 Nov 28;28(24):9515–9520. doi: 10.1021/bi00450a040. [DOI] [PubMed] [Google Scholar]
- DiGiuseppe J. A., Hunting D. J., Dresler S. L. Aphidicolin-sensitive DNA repair synthesis in human fibroblasts damaged with bleomycin is distinct from UV-induced repair. Carcinogenesis. 1990 Jun;11(6):1021–1026. doi: 10.1093/carcin/11.6.1021. [DOI] [PubMed] [Google Scholar]
- Drewinko B., Patchen M., Yang L. Y., Barlogie B. Differential killing efficacy of twenty antitumor drugs on proliferating and nonproliferating human tumor cells. Cancer Res. 1981 Jun;41(6):2328–2333. [PubMed] [Google Scholar]
- Eckardt-Schupp F., Siede W., Game J. C. The RAD24 (= Rs1) gene product of Saccharomyces cerevisiae participates in two different pathways of DNA repair. Genetics. 1987 Jan;115(1):83–90. doi: 10.1093/genetics/115.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg E. C. Deoxyribonucleic acid repair in the yeast Saccharomyces cerevisiae. Microbiol Rev. 1988 Mar;52(1):70–102. doi: 10.1128/mr.52.1.70-102.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin M. E., Epner S., Lasky L., Freedberg I. M. Bleomycin: effects upon mammalian epidermal DNA synthesis. Acta Derm Venereol. 1977;57(5):373–378. [PubMed] [Google Scholar]
- Hannan M. A., Nasim A. Genetic activity of bleomycin: differential effects on mitotic recombination and mutations in yeast. Mutat Res. 1978 Jun;53(3):309–316. doi: 10.1016/0165-1161(78)90003-1. [DOI] [PubMed] [Google Scholar]
- Henriques J. A., Brendel M. The role of PSO and SNM genes in DNA repair of the yeast Saccharomyces cerevisiae. Curr Genet. 1990 Dec;18(5):387–393. doi: 10.1007/BF00309906. [DOI] [PubMed] [Google Scholar]
- Ho K. S. Induction of DNA double-strand breaks by X-rays in a radiosensitive strain of the yeast Saccharomyces cerevisiae. Mutat Res. 1975 Dec;30(3):327–334. [PubMed] [Google Scholar]
- Jentsch S., McGrath J. P., Varshavsky A. The yeast DNA repair gene RAD6 encodes a ubiquitin-conjugating enzyme. Nature. 1987 Sep 10;329(6135):131–134. doi: 10.1038/329131a0. [DOI] [PubMed] [Google Scholar]
- Jones J. S., Prakash L. Transcript levels of the Saccharomyces cerevisiae DNA repair gene RAD18 increase in UV irradiated cells and during meiosis but not during the mitotic cell cycle. Nucleic Acids Res. 1991 Feb 25;19(4):893–898. doi: 10.1093/nar/19.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keszenman-Pereyra D. Repair of UV-damaged incoming plasmid DNA in Saccharomyces cerevisiae. Photochem Photobiol. 1990 Mar;51(3):331–342. doi: 10.1111/j.1751-1097.1990.tb01719.x. [DOI] [PubMed] [Google Scholar]
- Kimler B. F. The effect of bleomycin and irradiation on G2 progression. Int J Radiat Oncol Biol Phys. 1979 Sep;5(9):1523–1526. doi: 10.1016/0360-3016(79)90763-6. [DOI] [PubMed] [Google Scholar]
- Lawrence C. W., Christensen R. B. Metabolic suppressors of trimethoprim and ultraviolet light sensitivities of Saccharomyces cerevisiae rad6 mutants. J Bacteriol. 1979 Sep;139(3):866–876. doi: 10.1128/jb.139.3.866-876.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madura K., Prakash S., Prakash L. Expression of the Saccharomyces cerevisiae DNA repair gene RAD6 that encodes a ubiquitin conjugating enzyme, increases in response to DNA damage and in meiosis but remains constant during the mitotic cell cycle. Nucleic Acids Res. 1990 Feb 25;18(4):771–778. doi: 10.1093/nar/18.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C. W. Bleomycin-induced DNA repair by Saccharomyces cerevisiae ATP-dependent polydeoxyribonucleotide ligase. J Bacteriol. 1988 Oct;170(10):4991–4994. doi: 10.1128/jb.170.10.4991-4994.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C. W. Bleomycin-induced mutation and recombination in Saccharomyces cerevisiae. Mutat Res. 1978 Sep;58(1):41–49. doi: 10.1016/0165-1218(78)90094-0. [DOI] [PubMed] [Google Scholar]
- Moore C. W. Control of in vivo (cellular) phleomycin sensitivity by nuclear genotype, growth phase, and metal ions. Cancer Res. 1982 Mar;42(3):929–933. [PubMed] [Google Scholar]
- Moore C. W. Further characterizations of bleomycin-sensitive (blm) mutants of Saccharomyces cerevisiae with implications for a radiomimetic model. J Bacteriol. 1991 Jun;173(11):3605–3608. doi: 10.1128/jb.173.11.3605-3608.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C. W., Little J. B. Rapid and slow DNA rejoining in nondividing human diploid fibroblasts treated with bleomycin and ionizing radiation. Cancer Res. 1985 May;45(5):1982–1986. [PubMed] [Google Scholar]
- Moore C. W. Responses of radiation-sensitive mutants of Saccharomyces cerevisiae to lethal effects of bleomycin. Mutat Res. 1978 Aug;51(2):165–180. doi: 10.1016/s0027-5107(78)80016-5. [DOI] [PubMed] [Google Scholar]
- Mousstacchi E., Favaudon V. Cytotoxic and mutagenic effects of neocarzinostatin in wild-type and repair-deficient yeasts. Mutat Res. 1982 Apr;104(1-3):87–94. doi: 10.1016/0165-7992(82)90125-7. [DOI] [PubMed] [Google Scholar]
- Moustacchi E., Favaudon V., Bisagni E. Likelihood of the new antitumoral drug 10-[gamma-diethylaminopropylamino]-6-methyl-5H-pyrido[3',4':4,5]pyrrolo [2,3-g]isoquinoline (BD-40), a pyridopyrroloisoquinoline derivative, to induce DNA strand breaks in vivo and its nonmutagenicity in yeast. Cancer Res. 1983 Aug;43(8):3700–3706. [PubMed] [Google Scholar]
- Nunes E., Brum G., Candreva E. C., Schenberg Frascino A. C. Common repair pathways acting upon U.V.- and X-ray induced damage in diploid cells of Saccharomyces cerevisiae. Int J Radiat Biol Relat Stud Phys Chem Med. 1984 Jun;45(6):593–606. doi: 10.1080/09553008414550861. [DOI] [PubMed] [Google Scholar]
- Ozenberger B. A., Roeder G. S. A unique pathway of double-strand break repair operates in tandemly repeated genes. Mol Cell Biol. 1991 Mar;11(3):1222–1231. doi: 10.1128/mcb.11.3.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povirk L. F., Houlgrave C. W. Mutagenesis of bleomycin-damaged lambda phage in SOS-deficient and repair endonuclease-deficient Escherichia coli. Environ Mol Mutagen. 1988;11(4):461–472. doi: 10.1002/em.2850110407. [DOI] [PubMed] [Google Scholar]
- Prakash L. Characterization of postreplication repair in Saccharomyces cerevisiae and effects of rad6, rad18, rev3 and rad52 mutations. Mol Gen Genet. 1981;184(3):471–478. doi: 10.1007/BF00352525. [DOI] [PubMed] [Google Scholar]
- Resnick M. A., Martin P. The repair of double-strand breaks in the nuclear DNA of Saccharomyces cerevisiae and its genetic control. Mol Gen Genet. 1976 Jan 16;143(2):119–129. doi: 10.1007/BF00266917. [DOI] [PubMed] [Google Scholar]
- Robinson G. W., Nicolet C. M., Kalainov D., Friedberg E. C. A yeast excision-repair gene is inducible by DNA damaging agents. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1842–1846. doi: 10.1073/pnas.83.6.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl R. H., Prakash S., Prakash L. The SRS2 suppressor of rad6 mutations of Saccharomyces cerevisiae acts by channeling DNA lesions into the RAD52 DNA repair pathway. Genetics. 1990 Apr;124(4):817–831. doi: 10.1093/genetics/124.4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D., Zampetti-Bosseler F. Relationships between chromosome damage, cell cycle delay and cell killing induced by bleomycin or X-rays. Mutat Res. 1985 Aug;151(1):83–88. doi: 10.1016/0027-5107(85)90186-1. [DOI] [PubMed] [Google Scholar]
- Severgnini A., Lillo O., Nunes E. Analysis of bleomycin-induced mutagenic functions related to the PSO4 (= xs9) gene of Saccharomyces cerevisiae. Environ Mol Mutagen. 1991;18(2):102–106. doi: 10.1002/em.2850180204. [DOI] [PubMed] [Google Scholar]
- Sidik K., Smerdon M. J. Nucleosome rearrangement in human cells following short patch repair of DNA damaged by bleomycin. Biochemistry. 1990 Aug 14;29(32):7501–7511. doi: 10.1021/bi00484a020. [DOI] [PubMed] [Google Scholar]
- Siede W. The RAD6 gene of yeast: a link between DNA repair, chromosome structure and protein degradation? Radiat Environ Biophys. 1988;27(4):277–286. doi: 10.1007/BF01209756. [DOI] [PubMed] [Google Scholar]
- Steighner R. J., Povirk L. F. Bleomycin-induced DNA lesions at mutational hot spots: implications for the mechanism of double-strand cleavage. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8350–8354. doi: 10.1073/pnas.87.21.8350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steighner R. J., Povirk L. F. Effect of in vitro cleavage of apurinic/apyrimidinic sites on bleomycin-induced mutagenesis of repackaged lambda phage. Mutat Res. 1990 Feb;240(2):93–100. doi: 10.1016/0165-1218(90)90012-q. [DOI] [PubMed] [Google Scholar]
- Sung P., Prakash L., Matson S. W., Prakash S. RAD3 protein of Saccharomyces cerevisiae is a DNA helicase. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8951–8955. doi: 10.1073/pnas.84.24.8951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung P., Prakash L., Weber S., Prakash S. The RAD3 gene of Saccharomyces cerevisiae encodes a DNA-dependent ATPase. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6045–6049. doi: 10.1073/pnas.84.17.6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. M., Rosney C. M., Campbell J. B. Unusual sensitivity of ataxia telangiectasia cells to bleomycin. Cancer Res. 1979 Mar;39(3):1046–1050. [PubMed] [Google Scholar]
- Umezawa H., Maeda K., Takeuchi T., Okami Y. New antibiotics, bleomycin A and B. J Antibiot (Tokyo) 1966 Sep;19(5):200–209. [PubMed] [Google Scholar]


