Abstract
Anandamide (N-arachidonoylethanolamine) is an endogenous ligand for both the brain-type (CB1-R) and spleen-type (CB2-R) cannabinoid receptors. This investigation demonstrates that the periimplantation mouse uterus contains the highest levels of anandamide (142–1345 pmol/μmol lipid P; 1–7 μg/g wet weight) yet discovered in a mammalian tissue. The levels fluctuate with the state of pregnancy; down-regulation of anandamide levels is associated with uterine receptivity, while up-regulation is correlated with uterine refractoriness to embryo implantation. Anandamide levels are highest during the nonreceptive phase in the pseudopregnant uterus and in the interimplantation sites, and lowest at the site of embryo implantation. The lower levels of uterine anandamide at the implantation sites may be a mechanism by which implanting embryos protect themselves from the detrimental effects of this endogenous ligand. We also observed a reduced rate of zona-hatching of blastocysts in vitro in the presence of anandamide, and inhibition of implantation by systemic administration of a synthetic cannabinoid agonist CP 55,940. These adverse effects were reversed by SR141716A, a specific CB1-R antagonist. Taken together, the results suggest that an aberrant synthesis of anandamide and/or expression of the cannabinoid receptors in the uterus/embryo may account for early pregnancy failure or female infertility.
Because cannabinoids exert a broad array of central and peripheral effects (1) including adverse effects on pregnancy and embryonic development (2–5), the widespread use of marijuana is a serious concern to society. The mechanisms by which cannabinoids exert these diverse effects were not understood until the recent identification and cloning of G protein (Gi)-coupled cannabinoid receptors in the brain (CB1-R) and spleen (CB2-R). It is now apparent that many of the central and peripheral effects of cannabinoids are mediated via these receptors (6–8). Furthermore, the identification of a putative endogenous cannabinoid ligand, anandamide [N-arachidonoylethanolamine; 20:4 N-acylethanolamine (NAE)], in the brain suggested that cannabinoid ligand-receptor signaling could be operative under normal conditions in the central nervous system (9, 10), even though its significance is not yet understood.
We recently discovered that the CB1-R gene is expressed in the periimplantation mouse uterus and embryo, and the levels of CB1-R in the embryo, are much higher than those in the brain (11–13). Also, activation of CB1-R by cannabinoid ligands including anandamide interferes with preimplantation embryo development, and this effect is completely reversed by a specific CB1-R antagonist (13). These results and anandamide synthetic capacity of the periimplantation mouse uterus (11, 12, 14) suggested that cannabinoid-ligand receptor signaling during implantation could be physiologically and pharmacologically important.
Implantation is a process in which the embryo makes close physical and physiological contact with the maternal endometrium for the establishment of pregnancy. The fundamental feature of this process is the synchronized development of the embryo to the activated state of the blastocyst, its escape from the zona pellucida, and differentiation of the uterus to the receptive state (15, 16). This results in “two-way” interactions between the blastocyst and the uterus to initiate the attachment reaction that occurs at 2200–2300 h on day 4 of pregnancy in the mouse (17). This is followed by stromal cell decidualization at the sites of blastocysts (18). In this species, uterine environment with respect to implantation is divided into prereceptive, receptive, and nonreceptive (refractory) phases (15, 16, 19). The “window” of uterine receptivity for implantation occurs only for a limited period during pregnancy or pseudopregnancy (16). In the pregnant or pseudopregnant mouse, the prereceptive uterus on day 3 becomes receptive on day 4 (the day of implantation), while by day 5 (as examined by blastocyst transfers in uteri of pseudopregnant or oviduct-ligated uteri of pregnant mice), the uterus becomes refractory and fails to respond to the attachment reaction (16). In addition, the nonreceptive uterus becomes hostile to blastocyst survival (15, 16). The molecular mechanisms by which various uterine phases are achieved are not known. Because of the presence of CB1-R in the uterus and embryo and because of anandamide’s dramatic adverse effects on embryo development (13), we examined whether the uterus could be a major source of anandamide that could be associated with various phases of uterine receptivity.
MATERIALS AND METHODS
Animals and Tissue Preparation.
CD-1 mice (Charles River Breeding Laboratories) were housed in the animal care facility at the University of Kansas Medical Center according to National Institutes of Health and institutional guidelines on the care and use of laboratory animals. Adult female mice (20–25 g, 48–60 days old) were mated with fertile or vasectomized males of the same strain to produce pregnancy or pseudopregnancy, respectively. The morning of finding a vaginal plug was designated day 1 of pregnancy or pseudopregnancy. Normally, mice were killed between 0830–0900 h on various days of pregnancy or pseudopregnancy. To collect uteri shortly after the initiation of the attachment reaction, mice were killed at 0830–0930 h on day 5 of pregnancy. Early implantation sites on this day were visualized by intravenous injections (0.1 ml/mouse) of a Chicago Blue B dye solution (1% in saline). Mice were sacrificed 5 min later and implantation sites were identified as discrete areas of more intense blue (15, 16). On day 7, implantation sites were visible as distinct swellings and did not require any manipulation for their identification. Pseudopregnant mice produced by mating with vasectomized males were also sacrificed on days 4 and 5 at 0830–0900 h to examine whether embryonic influences were operative. Mouse brain tissues were analyzed for comparison. Tissues were stored at −80°C until used.
Extraction and Isolation of N-Acylethanolamines (NAEs) and N-Acylphosphatidylethanolamines (NAPEs).
Mouse uterine tissues (0.1–0.5 g, from 1–4 mice) were homogenized in chloroform/methanol (2:1, by vol), and the lipid extracts were partitioned against 2.5% aqueous NaCl (20). Individual brains were homogenized in chloroform and the extracts were centrifuged. A mixture of internal standards was added during homogenization (21). The standards were as follows: N-acyl-1,1,2,2,-2H-ethanolamines with acyl chain lengths of 16:0, 17:0, 18:0, 18:1n-9, 18:2n-6, and 20:4n-6 (d4 NAEs, 1 μg each), and N-heptadecanoyl-1,2-diheptadecanoylphosphatidylethanolamine (17:0 NAPE, 2.7 nmol). The lipid extracts were taken to dryness under a stream of nitrogen and redissolved in 1 ml of chloroform/methanol (2:1, by vol). After aliquots of 10 and 50 μl were removed for measurement of lipid phosphorus, the extracts were concentrated again and applied to silica gel H TLC plates. Chromatograms were developed in a solvent system of chloroform/methanol/concentrated ammonium hydroxide (90:10:1, by vol). NAE and NAPE standards were spotted in a lane on one edge of the plate. After development, the plate was sprayed with 2′,7′-dichlorofluorescein (0.1% in ethanol) and viewed under ultraviolet light. Bands corresponding to mobility (Rf) of the NAE and NAPE standards were scraped into tubes containing 3 ml of chloroform/methanol/water (30:50:20, by vol). The tubes were centrifuged, the solvents decanted, and the silica gel reextracted twice with 2 ml of the same solvent mixture. Chloroform and water were added to the combined eluates to make a final ratio of chloroform/methanol/water of 8:4:3 (by vol). The tubes were vortex mixed and centrifuged, and the bottom layer was removed and taken to dryness under a stream of nitrogen.
Derivatization and Analysis.
The NAE fractions were derivatized with 30 μl of tert-butyldimethylchlorosilyl/imidazole reagent (tBDMS; Alltech Associates) at 85°C for 1 h (21). After cooling, the NAE-tBDMS derivatives were extracted into 50 μl of hexane for gas chromatography-mass spectrometry (GC-MS). The NAPE fractions were reacted with phospholipase A2 (Trimeresurus flavoviridis) and phospholipase D (Streptomyces chromofuscus) as described to release the NAE moiety (22). The d4 NAE standard mixture was added to the tubes before the phospholipase A2 reaction, and NAEs were subsequently purified by TLC on layers of silica gel H, developed in hexane/diethylether/acetone/glacial acetic acid (40:20:20:1, by vol), scraped off, eluted, and derivatized.
GC-MS analysis of NAE-tBDMS derivatives was done on a Hewlett–Packard model 5890 series II gas chromatograph equipped with electronic pressure control, model 7673 autosampler, and model 5972 mass selective detector. A 30-meter Hewlett–Packard HP-5 MS (5% phenylmethylsiloxane) capillary column was programmed from 150°C to 280°C at 50° per min with an initial hold of 1 min and a final hold of 9 min. The samples were injected in the splitless mode with a pressure pulse of 30 psi for the first minute. Thereafter, the helium carrier gas flow rate was kept constant at 0.8 ml/min. The injection port was held at 230°C and the detector at 280°C. The mass spectrometer ionization voltage was 70 eV. The (M-57) ions of NAE-tBDMS derivatives were measured by selected ion monitoring. Under these conditions, the retention times ranged from 7.5 min for 16:0 NAE to 11.2 min for 20:4n-6 NAE. Endogenous NAEs were quantitated by comparison of their (M-57) ions to those of the corresponding d4 NAEs (21). As little as 1 ng (2.9 pmol) of anandamide (20:4n-6 NAE) in the original sample can be detected and quantified. NAE concentration in pmol/sample was normalized by dividing by the μmol of total lipid phosphorus (23) found in the extract. Average lipid phosphorus was 15.6 ± 1.5 μmol/g wet weight. The 17:0 NAE derived from the NAPE internal standard was used to correct NAPE values for loss during isolation and derivatization.
Blastocyst Growth and Hatching in Vitro.
To study effects of anandamide on blastocyst growth and hatching in vitro, eight-cell embryos recovered on day 3 (1000–1030 h) were cultured in groups (10–12 embryos/group) for 84 h in 25 μl of Whitten’s medium (24) in the absence or presence of anandamide and/or SR141716A (25). SR141716A is a specific antagonist to CB1-R (13) and was generously provided by Sanofi Recherche (Montepellier, France). All test agents were dissolved in ethanol and diluted with Whitten’s medium. The final ethanol concentration was less than 0.1%. The control cultures contained the same concentration of ethanol. Embryonic development was monitored every 12 h. The number of blastocysts showing complete hatching or considerable protrusion from zona-pellucidae was recorded at the end of the culture period (25).
Blastocyst Implantation in Vivo.
To study whether an active cannabinoid can influence implantation, mice on day 1 of pregnancy were implanted subcutaneously with miniosmotic pumps (model 1007D, Alzet, Palo Alto, CA) for steady-state delivery of a synthetic cannabinoid CP 55,940 (20 μg/h) or CP 55,940 (20 μg/h) plus a CB1-R antagonist SR141716A (5 μg/h). CP 55,940 was generously supplied by Pfizer Diagnostics. On day 5 (0900 h), mice were examined for implantation sites by the blue dye method (15, 16). Recovered blastocysts from mice not showing implantation sites were examined for zona dissolution. CP 55,940 or SR141716A was dissolved in propylene glycol for loading miniosmotic pumps.
RESULTS AND DISCUSSION
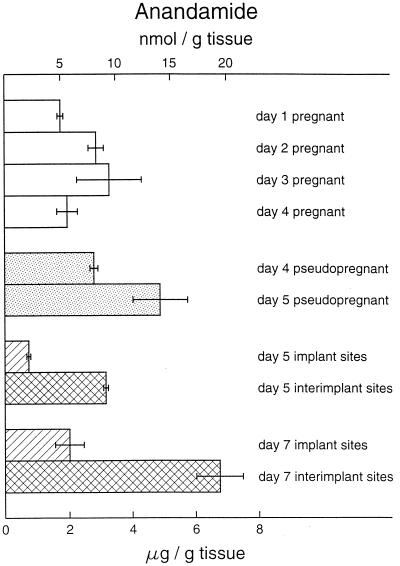
The present results (Table 1) indicate that mouse uterus contains very high levels of anandamide, reaching 1,345 pmol/μmol lipid P (20 nmol/g tissue) in the day 7 interimplantation sites (Fig. 1), whereas the brains of the same mice contained only 10–15 pmol/g tissue (data not shown). Anandamine levels in mammalian brain were previously found to be below 20 pmol/g tissue (21) but to vary somewhat depending on the region of the brain (26). Somewhat higher anandamide levels (30–100 pmol/g tissue) were reported for human brain (26) but the observed post mortem accumulation of anandamide and other NAEs in the central nervous system (21, 26, 27) has to be considered. In other organs, such as rat skin and spleen, and human heart and spleen, anandamide levels were found to be below 20 pmol/g tissue (26). Rat testes were recently shown to contain both NAEs and NAPEs with anandamide amounting to 6 pmol/g tissue (28). Mouse uterus therefore contains by far the highest levels of anandamide detected in any mammalian tissue. Furthermore, the mouse uterus is the only tissue identified so far in which anandamide is the primary component of NAE (75–95%). The changing levels of anandamide with changing pregnancy status are consistent with a possible role for this lipid molecule in early pregnancy. Higher uterine levels during the nonreceptive phase on day 5 of pseudopregnancy suggest that embryotoxic effects of the uterine environment during this time (15) could be due to increased anandamide levels. This suggestion is consistent with our observation that anandamide inhibits development of two-cell embryos to blastocysts in vitro (12, 13) and zona-hatching of blastocysts developed from eight-cell embryos in vitro (Table 2), and that these detrimental effects of anandamide are reversed by SR141716A. Furthermore, infusion of a synthetic cannabinoid ligand CP 55,940 via miniosmotic pumps during the preimplantation period prevented implantation in vivo when examined on day 5 (n = 5); most of the recovered blastocysts were zona-encased. These inhibitory effects were reversed with normal number of implantation sites (13.5 ± 1.2, n = 4) by coadministration of CP 55,940 with SR141716A, suggesting activation of CB1-R as mediator of the inhibitory effects of CP 55,940. Although CP 55,940 is more potent than Δ9-tetrahydrocannabinol, the major psychoactive component of marijuana, in other systems (29), these two cannabinoid ligands and anandamide are almost equipotent toward blastocyst CB1-R (13). Thus, remarkably lower levels of anandamide at the implantation sites compared with high levels at the interimplantation sites following the attachment reaction or in day 5 pseudopregnant uteri suggest that the implanting blastocysts regulate anandamide levels to protect against its detrimental effects.
Table 1.
Levels of endogenous NAEs in the periimplantation mouse uterus
| Day of Pregnancy | No. of determinations | Chain length:number of double bonds, pmol/μmol lipid phosphorus, mean ± SD
|
|||||
|---|---|---|---|---|---|---|---|
| 16:0 | 18:0 | 18:1n-9 | 18:1n-7 | 18:2n-6 | 20:4n-6 Anandamide* | ||
| 1 | 2 | 10.8 ± 1.2 | 11.0 ± 0.1 | 43.6 ± 21.9 | 1.3 ± 0.9 | 0.6 ± 0.4 | 289 ± 17.6 |
| 2 | 2 | 9.7 ± 0.0 | 11.5 ± 0.7 | 13.4 ± 0.1 | 1.8 ± 0.1 | 0.4 ± 0.4 | 428 ± 32.1 |
| 3 | 2 | 12.2 ± 1.3 | 13.4 ± 1.1 | 8.8 ± 2.2 | 3.2 ± 0.9 | 0.5 ± 0.3 | 566 ± 160.2 |
| 4 | 3 | 10.1 ± 0.7 | 8.8 ± 1.3 | 12.0 ± 3.7 | 1.9 ± 0.4 | 0.7 ± 0.4 | 345 ± 66.0 |
| 4, pseudopregnant | 2 | 11.6 ± 0.04 | 9.6 ± 0.7 | 10.6 ± 4.0 | 0.9 ± 0.6 | 0.8 ± 0.6 | 509 ± 12.2 |
| 5, pseudopregnant | 3 | 11.1 ± 1.0 | 11.6 ± 0.4 | 15.8 ± 4.3 | 1.9 ± 0.9 | 1.0 ± 0.4 | 937 ± 13.8 |
| 5, implant sites | 3 | 15.7 ± 0.03 | 7.5 ± 10.7 | 5.8 ± 0.3 | 1.9 ± 0.2 | 1.4 ± 0.03 | 142 ± 1.7 |
| 5, interimplant sites | 3 | 8.0 ± 1.1 | 11.6 ± 1.6 | 3.5 ± 0.8 | 2.1 ± 0.4 | 0.8 ± 0.1 | 583 ± 5.0 |
| 7, implant sites | 3 | 17.1 ± 0.2 | 38.1 ± 4.2 | 8.9 ± 1.3 | 4.1 ± 0.5 | 1.3 ± 0.5 | 330 ± 68.5 |
| 7, interimplant sites | 3 | 21.2 ± 0.9 | 18.1 ± 2.3 | 40.0 ± 12.0 | 1.4 ± 1.0 | 1.8 ± 0.9 | 1,345 ± 140.0 |
NAEs, isolated by TLC from lipid extracts of whole uteri, implantation sites, or interimplantation sites on indicated days of pseudopregnancy or pregnancy were analyzed by GC-MS. Levels of anandamide in day 5 pseudopregnant uteri were significantly higher (P < 0.05) from those on days 1–4 of pregnancy or pseudopregnancy (ANOVA followed by Student’s t-test). Level of anandamide in interimplantation sites were significantly higher (P < 0.05) than those from implantation sites (paired t-test).
Other polyunsaturated NAEs may also be present but were not included in this assay.
Figure 1.
Levels of endogenous NAEs in the periimplantation mouse uterus. NAEs were measured as described in the legend to Table 1. Levels of anandamide in day 5 pseudopregnant uteri were significantly different (P < 0.05) from those on days 1, 2, and 4 of pregnancy as well as from day 4 of pseudopregnancy (ANOVA followed by Student’s t test). Levels of anandamide in interimplantation sites were significantly higher (P < 0.05) than those from implantation sites (paired t test).
Table 2.
Effects of anandamide on blastocyst hatching in vitro
| Treatment | No. of embryos cultured | No. of embryos developed to blastocysts | Hatched blastocysts
|
|
|---|---|---|---|---|
| No. | % | |||
| Anandamide, nM | ||||
| 0 | 42 | 41 | 24 | 58.5 |
| 10 | 42 | 41 | 22 | 53.7 |
| 20 | 45 | 43 | 14 | 32.6* |
| Anandamide | 43 | 43 | 29 | 67.4 |
| (20 nM) plus SR (10 nM) | ||||
| SR, 10 nM | 31 | 31 | 21 | 67.7 |
Eight-cell embryos (10–12 embryos per group) were cultured in the presence or absence of anandamide and/or SR141716A (SR) for 84 h. Each experiment was repeated 3–4 times.
*P < 0.05 (χ2 test) compared with other groups.
The biosynthetic pathway and mechanism of anandamide synthesis remain an open question. Enzymatic condensation of free arachidonic acid with ethanolamine, independent of ATP and coenzyme A, has been shown to generate anandamide in preparations of rat, rabbit, and bovine brain (30–33), but the high Km values for both substrates make it possible that these results actually represent the activity of amidohydrolase (34) acting in reverse in the presence of high substrate concentrations (33, 34). There is also evidence that anandamide can be synthesized in neuronal cells (35) and rat testes (28) by phosphodiesterase-mediated cleavage of NAPEs, most likely by the transacylation-phosphodiesterase pathway involved in the biosynthesis of saturated and monounsaturated NAEs (reviewed in refs. 36 and 37). Very recent evidence strongly suggests that anandamide is synthesized in rat brain by the transacylation-phosphodiesterase pathway rather than by N-acylation of ethanolamine (38). However, our present results show that arachidonic acid is only a minor component of the N-acyl groups of NAPE in the mouse uterus during early pregnancy (Table 3), whereas the corresponding anandamide represents up to 95% of total NAEs (Table 1). Thus, biosynthesis of anandamide in the uterus by the N-acylation-phosphodiesterase pathway (36–38) would require either the action of a phosphodiesterase exhibiting extraordinary selectivity for the N-arachidonoyl moiety of NAPE, or the continuous selective degradation of NAEs other than anandamide. Therefore, direct N-acylation of ethanolamine must be considered as a possible alternative pathway of anandamide synthesis in this tissue. This is consistent with our findings of a relatively low substrate requirement (Km of 3.8 μM and 1.2 mM for arachidonic acid and ethanolamine, respectively) for the uterine anandamide synthase activity that was also shown to be active in the presence of phenylmethylsulfonyl fluoride, an inhibitor of the amidohydrolase (14). It is interesting to note that the changing uterine levels of anandamide with pregnancy status are reflected in changing patterns of uterine anandamide synthase and amidohydrolase activity (14).
Table 3.
Levels of NAPEs in the periimplantation mouse uterus
| Day of pregnancy | No. of determinations | Chain length:number of double bonds, pmol/μmol lipid phosphorus, mean ± SD
|
|||||
|---|---|---|---|---|---|---|---|
| 16:0 | 18:0 | 18:1n-9 | 18:1n-7 | 18:2n-6 | 20:4n-6 Anandamide* | ||
| 1 | 2 | 21.4 ± 0.4 | 10.4 ± 0.3 | 6.9 ± 0.4 | 4.9 ± 0.9 | 2.2 ± 1.6 | 1.5 ± 0.3 |
| 2 | 2 | 19.0 ± 0.6 | 11.2 ± 0.3 | 7.9 ± 0.3 | 3.9 ± 0.6 | 2.0 ± 1.4 | 1.7 ± 1.2 |
| 3 | 2 | 19.3 ± 6.0 | 17.8 ± 2.6 | 10.8 ± 2.0 | 4.9 ± 0.6 | 1.8 ± 1.2 | 2.1 ± 0.4 |
| 4 | 3 | 21.3 ± 0.3 | 15.0 ± 2.2 | 17.0 ± 5.6 | 4.3 ± 1.0 | 4.1 ± 1.7 | 2.0 ± 0.3 |
| 4, pseudopregnant | 2 | 22.0 ± 2.2 | 11.6 ± 0.9 | 6.5 ± 0.9 | 4.5 ± 0.2 | 2.6 ± 1.8 | 1.6 ± 0.03 |
| 5, pseudopregnant | 3 | 22.0 ± 3.7 | 14.0 ± 3.5 | 10.1 ± 2.7 | 4.0 ± 2.7 | 4.3 ± 2.0 | 1.9 ± 0.2 |
| 5, implant sites | 3 | 49.4 ± 3.0 | 29.2 ± 2.6 | 14.3 ± 2.1 | 6.5 ± 1.8 | 11.6 ± 1.9 | 5.1 ± 1.0 |
| 5, interimplant sites | 3 | 22.3 ± 3.4 | 17.3 ± 1.6 | 12.8 ± 3.5 | 6.9 ± 0.4 | 7.2 ± 1.1 | 2.7 ± 0.7 |
| 7, implant sites | 3 | 94.0 ± 22.6 | 117.2 ± 29.1 | 17.4 ± 2.0 | 17.3 ± 3.7 | 11.4 ± 5.7 | 5.2 ± 0.6 |
| 7, interimplant sites | 3 | 47.9 ± 1.5 | 34.0 ± 11.6 | 20.9 ± 1.9 | 6.0 ± 0.9 | 4.2 ± 2.1 | 11.9 ± 2.4 |
NAPEs isolated by TLC from lipid extracts of whole uteri, implantation sites, or interimplantation sites on indicated days of pseudopregnancy or pregnancy were reacted with phospholipase A2 and phospholipase D to generate NAEs. Levels of N-20:4 PE in interimplantation sites were significantly higher (P < 0.05) than those from implantation sites on day 7 of pregnancy (paired t-test).
Other polyunsaturated NAEs may also be present but were not included in this assay.
Because the levels of anandamide in the uterus are several orders of magnitude higher than those in the brain, the signaling function of this molecule during early pregnancy may be physiologically different from its role in the central nervous system. However, it may be questioned why anandamide, which is detrimental to embryonic development and implantation, is present in the uterus during the receptive phase and in implantation sites at higher levels than in other tissues. Because the uterus is composed of heterogeneous cell types, it could be that the local concentration of this molecule at the sites of blastocysts in the receptive uterus or at the implantation sites does not reach the required levels to affect embryonic functions and implantation. Information regarding cell-specific synthesis and hydrolysis of this molecule in the uterus will be required to address this issue. Another possibility is that anandamide serves as a reservoir of arachidonic acid for the generation of prostaglandins (PGs) which are important for embryo development and implantation (39–41). This is consistent with higher levels of amidohydrolase activity and of PGE2 in implantation sites (14, 41). Increases in uterine anandamide synthase activity with concomitant decreases in amidohydrolase activity by indomethacin in vivo and in vitro (14) also support this assumption. It should be noted that inhibition of PG synthesis by indomethacin interferes with implantation (40, 41). Alternatively, anandamide may be involved in regulating the “window” of implantation by synchronizing the embryo development (blastocyst formation, expansion, zona dissolution, and activation) with preparation of the uterus to the receptive state, since this lipid mediator via activation of CB1-R can modulate adenylyl cyclase and Ca2+ channel activities, two important second messenger systems involved in various cellular functions (6, 10). On the other hand, excessive uterine levels of anandamide during early pregnancy may induce prematurely the nonreceptive phase of the uterus, thus disrupting embryo survival and establishment of pregnancy. Similar mechanisms may account for unexplained infertility in women (42). Reports of adverse effects of cannabinoid exposure on pregnancy (2–5) are consistent with the recent evidence for the presence of CB1-R receptor (43) and anandamide synthetic capacity in the human endometrium (unpublished data). Collectively, these results place the uterus and embryo as important and physiologically relevant targets for cannabinoid ligand-receptor signaling.
Acknowledgments
This work was supported in part by National Institutes of Health Grants DA06668, and as part of the National Cooperative Program on Markers of Uterine Receptivity for Blastocyst Implantation (Grant HD29968) and Grant HD12304 (to S.K.D.) and Grant GM45741 and by The Hormel Foundation (to H.H.O.S.).
ABBREVIATIONS
- NAE
N-acylethanolamine
- NAPE
N-acylphosphatidylethanolamine
- tBDMS
tert-butyldimethylchlorosilyl/imidazole reagent
- GC-MS
gas chromatography-mass spectrometry
References
- 1.Dewey W L. Pharmacol Rev. 1986;38:151–178. [PubMed] [Google Scholar]
- 2.Nahas G, Latour C. Med J Aust. 1992;156:495–497. [PubMed] [Google Scholar]
- 3.Rosenkrantz H. In: Marihuana: Biological Effects. Nahas G G, Paton W D M, editors. Oxford: Pergamon; 1979. pp. 479–499. [Google Scholar]
- 4.Dalterio S, Battke A. J Endocrinol. 1981;91:509–514. doi: 10.1677/joe.0.0910509. [DOI] [PubMed] [Google Scholar]
- 5.Asch R H, Smith C G. J Reprod Med. 1987;31:1071. [PubMed] [Google Scholar]
- 6.Howlett A C, Fleming R M. Mol Pharmacol. 1984;27:492–436. [PubMed] [Google Scholar]
- 7.Matsuda L A, Lolait S J, Brownstein M J, Young A C, Bonner T I. Nature (London) 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 8.Munro S, Thomas K L, Abu-Shaar M. Nature (London) 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 9.Devane W A, Hanus I, Breuer A, Pertwee R G, Stevenson L A, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 10.Felder C C, Briley E M, Axelrod J, Simpson J T, Mackie K, Devane W A. Proc Natl Acad Sci USA. 1993;90:7656–7660. doi: 10.1073/pnas.90.16.7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das S K, Paria B C, Chakraborty I, Dey S K. Proc Natl Acad Sci USA. 1995;92:4332–4336. doi: 10.1073/pnas.92.10.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paria B C, Das S K, Dey S K. Proc Natl Acad Sci USA. 1995;92:9460–9464. doi: 10.1073/pnas.92.21.9460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Z M, Paria B C, Dey S K. Biol Reprod. 1996;55:756–761. doi: 10.1095/biolreprod55.4.756. [DOI] [PubMed] [Google Scholar]
- 14.Paria B C, Deutsch D D, Dey S K. Mol Reprod Dev. 1996;45:183–192. doi: 10.1002/(SICI)1098-2795(199610)45:2<183::AID-MRD11>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 15.Psychoyos A. In: Handbook of Physiology. Greep R O, Astwood E G, editors. Washington, DC: Am. Physiol. Soc.; 1973. pp. 187–215. [Google Scholar]
- 16.Paria B C, Huet-Hudson Y M, Dey S K. Proc Natl Acad Sci USA. 1993;90:10159–10162. doi: 10.1073/pnas.90.21.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das S K, Wang X-N, Paria B C, Damm D, Abraham J A, Klagsbrun M, Andrews G K, Dey S K. Development (Cambridge, UK) 1994;120:1071–1083. doi: 10.1242/dev.120.5.1071. [DOI] [PubMed] [Google Scholar]
- 18.Huet Y M, Andrews G K, Dey S K. Endocrinology. 1989;125:1683–1690. doi: 10.1210/endo-125-3-1683. [DOI] [PubMed] [Google Scholar]
- 19.Yoshinaga K. In: Progress in Reproductive Biology. Leroy F, Finn C A, Psychoyos A, Hubinot P O, editors. Vol. 7. Basel: Krager; 1980. pp. 189–199. [Google Scholar]
- 20.Folch J, Lees M, Sloane Stanley G M. J Biol Chem. 1957;226:497–507. [PubMed] [Google Scholar]
- 21.Schmid P C, Krebsbach R J, Perry S R, Dettmer T M, Maasson J L, Schmid H H O. FEBS Lett. 1995;375:117–120. doi: 10.1016/0014-5793(95)01194-j. 125–126. [DOI] [PubMed] [Google Scholar]
- 22.Schmid P C, Natarajan V, Weis B K, Schmid H H O. Chem Phys Lipids. 1986;41:195–207. doi: 10.1016/0009-3084(86)90022-8. [DOI] [PubMed] [Google Scholar]
- 23.Bartlett G R. J Biol Chem. 1959;234:466–468. [PubMed] [Google Scholar]
- 24.Whitten W K. Adv Biosci. 1971;6:129–139. [Google Scholar]
- 25.Paria B C, Dey S K. Proc Natl Acad Sci USA. 1990;87:4756–4760. doi: 10.1073/pnas.87.12.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Felder C C, Nielsen A, Briley E M, Palkovits M, Priller J, Axelrod J, Nguyen D N, Richardson J M, Riggin R M, Koppel G A, Paul S M, Becker G W. FEBS Lett. 1996;393:231–235. doi: 10.1016/0014-5793(96)00891-5. [DOI] [PubMed] [Google Scholar]
- 27.Kempe K, Hsu F-F, Bohrer A, Turk J. J Biol Chem. 1996;271:17287–17295. doi: 10.1074/jbc.271.29.17287. [DOI] [PubMed] [Google Scholar]
- 28.Sugiura T, Konda S, Sukagawa A, Tonegawa A, Nakane S, Yamashita A, Waku K. Biochem Biophys Res Commun. 1996;218:113–117. doi: 10.1006/bbrc.1996.0020. [DOI] [PubMed] [Google Scholar]
- 29.Herkenham M, Lynn A B, Little M D, Johnson M R, Melvin L S, De Costa B R, Rice K C. Proc Natl Acad Sci USA. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deutsch D D, Chin S A. Biochem Pharmacol. 1993;46:791–796. doi: 10.1016/0006-2952(93)90486-g. [DOI] [PubMed] [Google Scholar]
- 31.Kruszka K K, Gross R W. J Biol Chem. 1994;269:14345–14348. [PubMed] [Google Scholar]
- 32.Devane W A, Axelrod J. Proc Natl Acad Sci USA. 1994;91:6698–6701. doi: 10.1073/pnas.91.14.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ueda N, Kurahashi Y, Yamamoto S, Tokunaga T. J Biol Chem. 1995;270:23823–23827. doi: 10.1074/jbc.270.40.23823. [DOI] [PubMed] [Google Scholar]
- 34.Schmid P C, Zuzarte-Augustin M L, Schmid H H O. J Biol Chem. 1985;260:14145–14149. [PubMed] [Google Scholar]
- 35.Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz J-C, Piomelli D. Nature (London) 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- 36.Schmid H H O, Schmid P C, Natarajan V. Prog Lipid Res. 1990;29:1–43. doi: 10.1016/0163-7827(90)90004-5. [DOI] [PubMed] [Google Scholar]
- 37.Schmid H H O, Schmid P C, Natarajan V. Chem Phys Lipids. 1996;80:133–142. doi: 10.1016/0009-3084(96)02554-6. [DOI] [PubMed] [Google Scholar]
- 38.Sugiura T, Kondo S, Sukagawa A, Tonegawa T, Nakane S, Yamashita A, Ishima Y, Waku K. Eur J Biochem. 1996;240:55–62. doi: 10.1111/j.1432-1033.1996.0053h.x. [DOI] [PubMed] [Google Scholar]
- 39.Biggers J D, Leonov B V, Baskar J, Fried J. Biol Reprod. 1978;19:519–533. doi: 10.1095/biolreprod19.3.519. [DOI] [PubMed] [Google Scholar]
- 40.Gupta A, Huet Y M, Dey S K. Endocrinology. 1989;124:546–547. doi: 10.1210/endo-124-1-546. [DOI] [PubMed] [Google Scholar]
- 41.Kennedy T J. Biol Reprod. 1977;16:286–291. doi: 10.1095/biolreprod16.3.286. [DOI] [PubMed] [Google Scholar]
- 42.Morell V. Science. 1995;269:775–777. doi: 10.1126/science.7638588. [DOI] [PubMed] [Google Scholar]
- 43.Galiegue S, Mary S, Marchand J, Dussossay D, Carriere D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]